研究業績
研究業績一覧へ
発表論文
2021年
-
“Alkyl decorated metal–organic frameworks for selective trapping of ethane from ethylene above ambient pressures”
Andreas Schneemann, Yuan Jing, Jack D. Evans, Takashi Toyao, Yuh Hijikata, Yuichi Kamiya, Ken-ichi Shimizu, Nicholas C. Burtcha, Shin-ichiro Noro
Dalton Transactions, 2021, 50, 10423-10435.
DOI:10.1039/D1DT01477C
-
“Elucidation of detailed pore size distribution of (NH4)4SiW12O40 sponge crystal”
Kaito Matsuda, Yuto Kobayashi, Sayaka Inoue, Yusuke Morita, Tomoya Ishikawa, Takeshi Uyama, Takeru Ito, Yuichi Kamiya, Kei Inumaru
Chemistry Letters, 2021, 50, 1736-1739.
DOI:10.1246/cl.210246
-
“Oxidative decomposition of ammonium ion with ozone in the presence of cobalt ion and chloride ion toward treatment of radioactive liquid waste”
Haruka Aihara, Sou Watanabe, Atsuhiro Shibata, Ryoichi Otomo, Yuichi Kamiya
Progress in Nuclear Energy, 2021, 139, 103872
DOI:10.1016/j.pnucene.2021.103872
Abstract
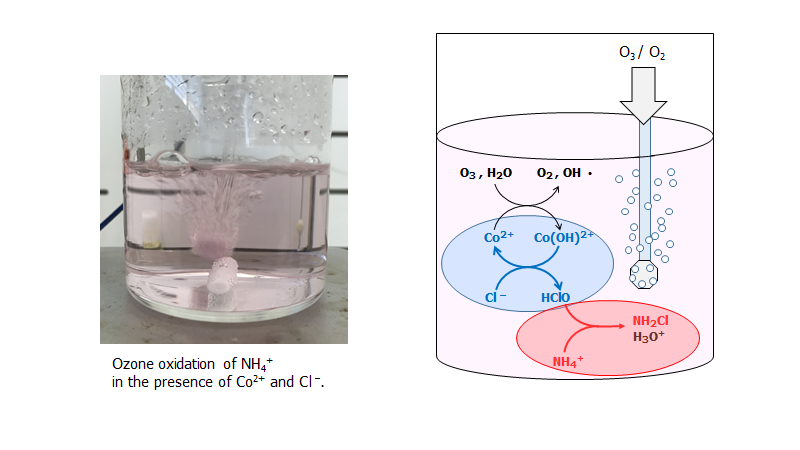
To prevent unexpected accidents at nuclear facilities caused by accumulated ammonium nitrate in an aqueous liquid waste containing ammonium salts and nitric acid, NH4+ in the liquid waste must be decomposed under mild reaction conditions. In this study, we investigated the oxidative decomposition of NH4+ with O3 at 333 K in the presence of a homogeneous Co2+ catalyst and Cl– in the wide pH range of the test solution. The reaction behavior was greatly affected by pH of the test solution. In a basic solution at pH 12, high conversion of NH4+ was obtained even in the absence of Co2+ and Cl–, and the main product was NO3-. However, Co2+ and Cl– in the solution greatly enhanced the decomposition rate of NH4+ in acidic to mild basic solutions (pH 1–8), while only low conversion of NH4+ was observed unless both Co2+ and Cl– were present. For the reaction with Co2+ and Cl– in the solutions, NH4+ was transformed mainly into chloramines (NHxCl3x, x =1–3) by the reaction with HClO, which was formed by the reaction of Cl– with O3 catalyzed by the homogeneous Co2+ catalyst, and led to the high decomposition rate of NH4+. Cl– suppressed the formation of the precipitate CoO(OH) during the reaction and consequently the Co2+ catalyst stably existed in the reaction solution, which was another reason for the high decomposition rate of NH4+ in the presence of Cl–. Owing to the swift decomposition of NH4+ under mild reaction conditions and small formation of secondary waste, the oxidative decomposition of NH4+ in the presence of the homogeneous Co2+ catalyst and Cl– is suitable and applicable for the treatment of the aqueous liquid waste containing ammonium salts and nitric acid.
-
“Ultrahigh-Pressure Preparation and Catalytic Activity of MOF-Derived Cu Nanoparticles”
Ichiro Yamane, Kota Sato, Ryoichi Otomo, Takashi Yanase, Akira Miura, Taro Nagahama, Yuichi Kamiya and Toshihiro Shimada
Nanomaterials, 2021,11(4),1040; DOI:10.3390/nano11041040
-
“Ceria-supported palladium as a highly active and selective catalyst for oxidative decomposition of ammonium ion in water with ozone”
Philip Anggo Krisbiantoro, Tomokazu Togawa, Koki Kato, Jieqiong Zhang, Ryoichi Otomo, Yuichi Kamiya
Catal. Commun., 2021, 149, 106204; DOI:10.1016/j.catcom.2020.106204
Abstract
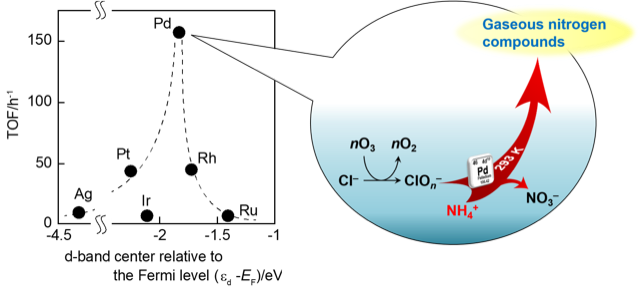
Al2O3-supported precious metal catalysts were tested for the oxidative decomposition of NH4+in water with O3and the effects of support on the catalytic activity of Pd were investigated. Pd/CeO2was found to be the best catalyst in terms of activity, selectivity and stability and was more active and selective than Co3O4, which is one of the best catalysts reported so far. The reaction proceeded only in the presence of Cl–. The moderate value of the d-band center relative to the Fermi level of Pd was found to be favorable for the activation of reactants, leading to a high catalytic performance.
学会発表
2021年度
- 化学系学協会北海道支部2022年冬季研究発表会(1月25日(火)~26日(水),北海道)
(口頭発表) 閻“Removal and reduction of nitrate over anion-exchange resin incorporating gold nanoparticle toward purification of groundwater polluted with nitrate”
- 化学系学協会北海道支部2022年冬季研究発表会(1月25日(火)~26日(水),北海道)
(口頭発表) 徐”水中硝酸イオン還元をアンモニアへと高選択還元する担持ニッケル触媒への白金の添加効果”
- 化学系学協会北海道支部2022年冬季研究発表会(1月25日(火)~26日(水),北海道)
(口頭発表)秋山”フラックス法によるFe-Sn系ペロブスカイトの合成”
- The 4th International Conference on Chemical Sciences (11月29-30日,オンライン)
(招待講演) 神谷 “Catalytic and photocatalytic remediation of groundwater polluted with nitrate”
- 18th Japan-Korea Symposium on Catalysis (18JKSC) (11月23-25日,オンライン)
(招待講演) 神谷 “Catalytic and photocatalytic reduction of nitrate in water for purification of polluted groundwater”
- 1st Japan-China Symposium on Catalysis (1stJCSC) (10月10日-12日,オンライン)
(口頭発表) 黄 “Application of water-resistant MOF for catalytic reduction of nitrite and nitrate in water”
- 2021年度JPIJS講演会 (11月13日,函館)
(招待講演) 大友 “異元素置換によるFe系ペロブスカイト型酸化物の高機能化”
- 第51回石油・石油化学討論会 (11月11日-12日,函館)
(口頭発表) 岩本 “粒子径および形態の異なるTiO2からのTi2O3の合成”
- 第51回石油・石油化学討論会 (11月11日-12日,函館)
(口頭発表) 北川 “排ガスからのメタンスリップの抑制に向けたオゾンを酸化剤とする低温メタン燃焼触媒の探索”
- 第128回触媒討論会(9月15日-17日,オンライン)
(口頭発表) 田中“Ti2O3のアセタール化に対する触媒活性に前処理が及ぼす影響”
- The 3rd International Conference on Science, Mathematics, Environmental and Education (ICoSMEE)(7月27-28日,オンライン)
(招待講演)大友“Catalysis of metal oxide materials with less stable valence”
- 化学系学協会北海道支部2021年夏季研究発表会(7月17日,北海道 オンライン)
(口頭発表) 岩本“異なるTiO2原料を用いたTi2O3の合成”
表彰






