研究業績
研究業績一覧へ
発表論文
2020年
- “SrFe1-xSnxO3-δnanoparticles with enhanced redox properties for catalytic combustion of benzene”
Kazutaka Hashimoto, Ryoichi Otomo and Yuichi Kamiya
Catal. Sci. Technol. , 10, 2020, 6342-6349DOI:10.1039/D0CY01154
Abstract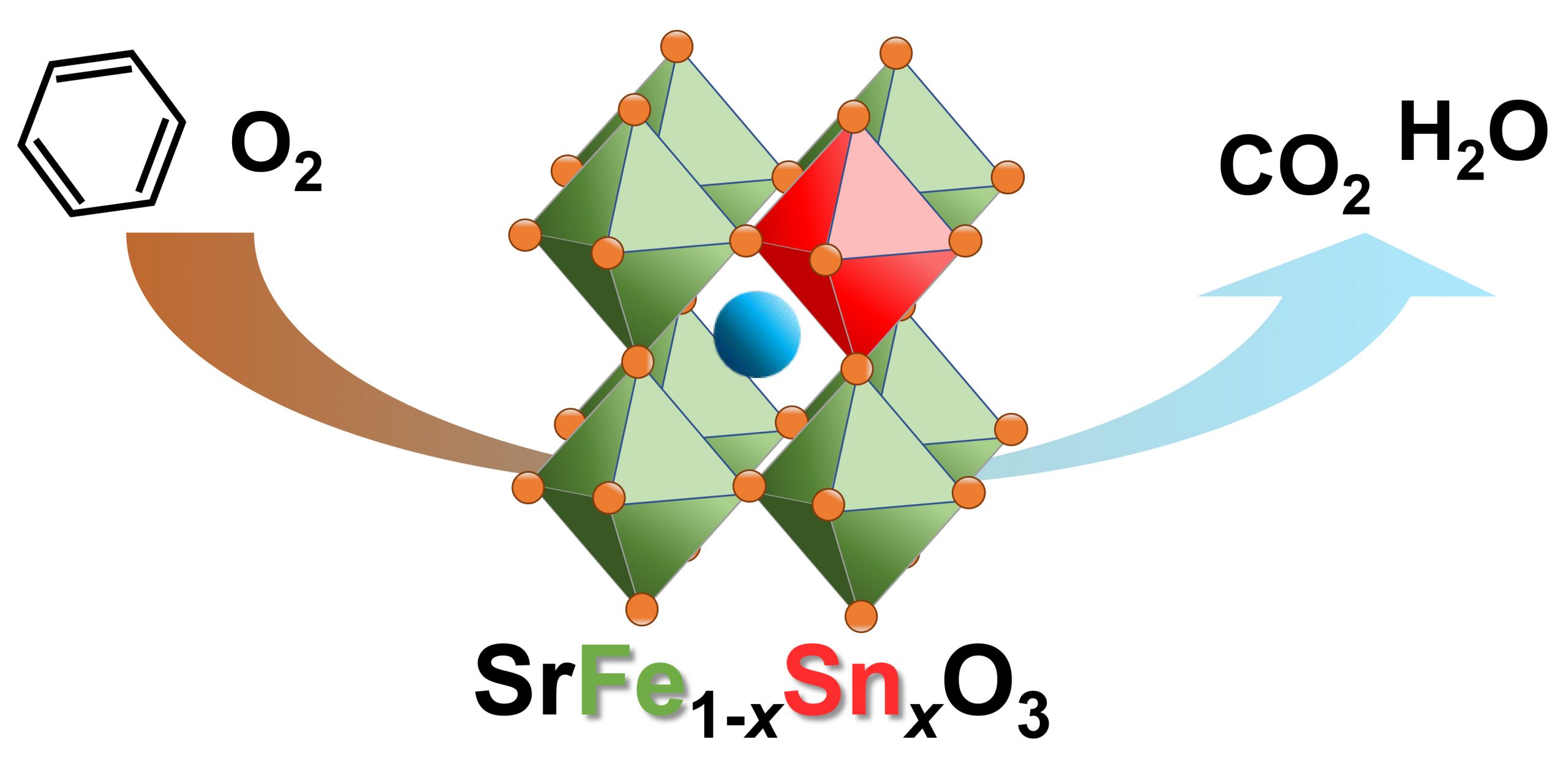
A series of Sn-substituted strontium ferrate SrFe1-xSnxO3-δ(x = 0, 0.25, 0.50, 0.75 and 1.0) were prepared by the polymerized complex method. Influence of the substitution of Fe in SrFeO3-δwith Sn on structural, redox, and catalytic properties was investigated. X-ray diffraction and 57Fe Mossbauer analyses revealed that the partial substation of Fe with Sn expanded the lattice of the perovskite-type structure, leading to elongation of Fe-O bonds. The partial substitution resulted in the decline of the particle size and the increase of specific surface area. H2-TPR and TG measurements in H2or O2flow indicated that the partial substitution significantly accelerated the redox rates of Fe at 500 °C. Due to the increased surface area and enhanced redox properties, the partially substituted SrFe1-xSnxO3-δshowed higher catalytic activity than SrFeO3-δfor combustion of benzene.
- “Chitosan-functionalized natural magnetic particle@silica modified with (3-chloropropyl) trimethoxysilane as a highly stable magnetic adsorbent for gold(III) ion”
- Nuryono Nuryono, Dikki Miswanda, Satya Candra Wibawa Sakti, Bambang Rusdiarso, Philip Anggo Krisbiantoro, Ryoichi Otomo, Yuichi Kamiya
Mater. Chem. Phys.
, 255, 2020, 123507-123517
DOI:10.1016/j. matchemphys. 2020. 123507
Abstract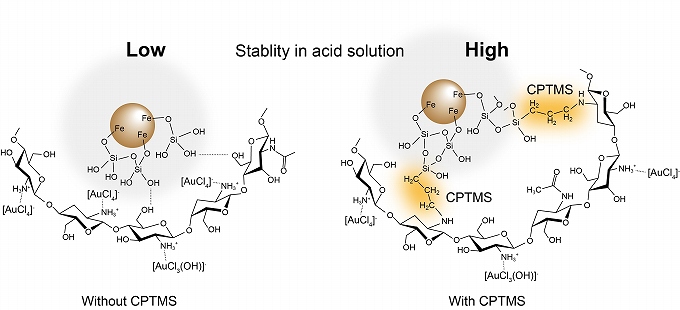
It is keenly desired to develop an environmentally benign and highly stable magnetic adsorbent for efficient recovery of Au(III) ion from the solution. In the present study, we investigated the synthesis of magnetic adsorbents that the magnetic particles, which was prepared from natural iron sand, were modified with silica layer on which chitosan was fixed through 3-chloropropyltrymethoxysilane (CPTMS) by a sol-gel process. Since the magnetic particles were almost completely covered with the silica layer and chitosan was tightly fixed on it through CPTMS, the adsorbent was highly stable in an acidic solution with pH 3 or lower. Excess CPTMS significantly lowered the adsorption capacity for Au(III) ion and lead to little improvement in the stability. Thus, 1 mmol of CPTMS against 4 mmol of chitosan gave the best magnetic adsorbent in terms of stability and adsorption capacity, of which the maximum was 112 mg g?1for Au(III) ion at pH 5. The adsorbent showed high selectivity to Au(III) in the solution containing Cu(II) and Zn(II), and it was reusable at least two times with the reduction in the percentage of Au(III) recovery in each reuse was less than 17%. The magnetic adsorbent was separable from the solution simply with an external magnet, while the modification caused a slight decrease of the saturated magnetization.
- “Oxidation of Ammonia Nitrogen with Ozone in Water: A Mini Review”
Philip Anggo Krisbiantoro, Koki Kato, Lina Mahardiani, Yuichi Kamiya
J. Ind. Chem. Soc., 2020, 3, 17-27DOI:10.34311/jics.2020.03.1.17
Abstract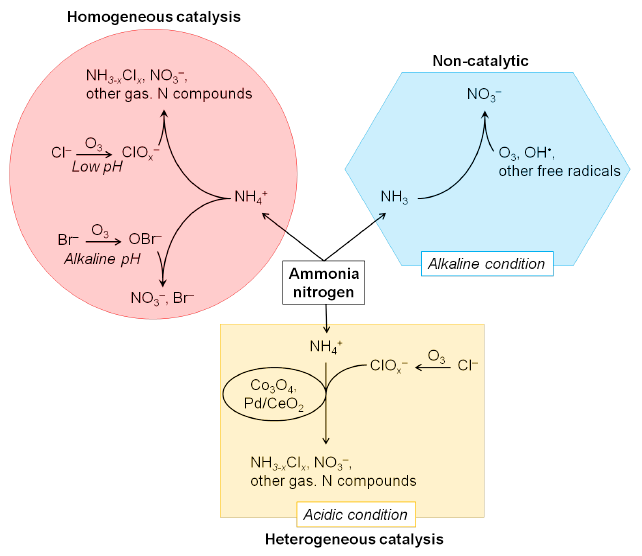
Since ammonia nitrogen is a pollutant causing eutrophication, it must be removed from wastewater to develop a sustainable environment and society. Ozonation, which is an oxidation reaction with ozone, is an effective and efficient method for the removal of ammonia nitrogen in wastewater because the reaction can proceed at low temperature and atmospheric pressure. Although the researches in ozonation of ammonia nitrogen have been going on for the last five decades, the reaction mechanism has not yet been well understood, and the papers focusing on the reaction mechanism are very few. In this short review paper, the progress in the oxidation of ammonia nitrogen with ozone both in non-catalytic and catalytic reactions is summarized to provide a better understanding of the reaction mechanism for ozonation of ammonia nitrogen in the water.
- “Phosphate recovery from an aqueous solution through adsorption-desorption cycle over thermally treated activated carbon”
- Toshiki Miyazato, Nuryono Nuryono, Mrina Kobune, Bambang Rusdiarso, Ryoichi Otomo, Yuichi Kamiya
J. Water Proc. Eng., 36 (2020) 101302DOI:10.1016/j.jwpe.2020.101302
Abstract
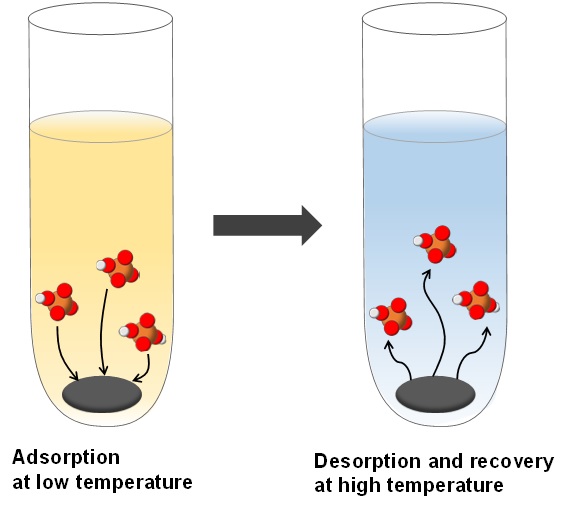
In the present study, we tested nine commercially available activated carbons for their abilities to recover phosphate ion from an aqueous solution by a temperature swing method, in which phosphate ion was adsorbed at 303 K, followed by desorption and recovery in pure water at 373 K. While the activated carbon made from coconut shell and manufactured by Nakarai Tesque Inc. (trade name: Charcoal Activated) had a moderate adsorption capacity at 303 K, little amount of phosphate ion was adsorbed on it at 373 K, meaning that it was an appropriate adsorbent for the temperature swing method. Since the adsorbed amounts of phosphate ion for various activated carbons at 303 K were correlated with the number of basic sites on them and were significantly increased as pH of the solution decreased, it is presumed that phosphate ion was adsorbed on the basic sites of the activated carbons with electrostatic interaction mediated by protons. High-temperature thermal treatment of the activated carbon in a vacuum increased the recovered amount of phosphate ion. This increase was brought about by elimination of oxygen-containing functional groups from the activated carbon. By using activated carbon obtained by the thermal treatment at 1273 K for 3 h, 86% of phosphate ion was recovered from an aqueous solution with 1.0 mmol L?1 of phosphate ion through the temperature swing between 303 and 373 K. The thermally treated activated carbon was reusable at least three times without any severe performance loss.
- “The role of cobalt oxide or magnesium oxide in ozonation of ammonia nitrogen in water”
Philip Anggo Krisbiantoro, Tomokazu Togawa, Lina Mahardiani, Haruka Aihara, Ryoichi Otomo, Yuichi Kamiya
Appl. Catal. A, 596 (2020), 117515DOI:10.1016/j.apcata.2020.117515
Abstract
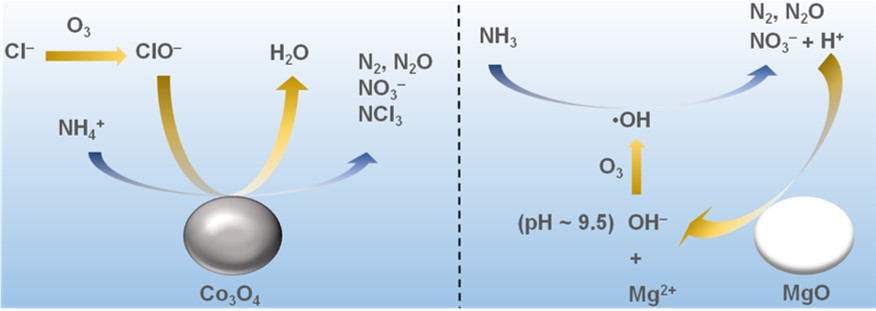
In this study, the reaction mechanisms for ozonation of ammonia nitrogen in the presence of Co3O4or MgO were investigated. For the reaction over Co3O4, Cl–in the reaction solution was indispensable and ClO–was formed by a non-catalytic oxidation of Cl–. Co3O4promoted the reaction of NH4+with ClO–to give the products including NO3–, chloramines and gaseous products. In contrast, Cl–was unnecessary for the reaction with MgO. pH of the reaction solution was maintained at around 9 throughout the reaction owing to partial dissolution of MgO. Ammonia nitrogen was decomposed to mainly NO3–by non-catalytic radical reaction involving OH・, which was formed by the reaction of OH–with O3in weakly basic solution. To keep the reaction solution weakly basic, H+formed with the decomposition of NH4+was neutralized. As a result, about the same amount of Mg2+as that of decomposed ammonia nitrogen was dissolved.
- “Catalytic reduction of nitrate in water over alumina-supported nickel catalyst toward purification of polluted groundwater”
Marina Kobune, Dai Takizawa, Jun Nojima, Ryoichi Otomo, Yuichi Kamiya
Catal. Today, 352 (2020), 204-211DOI:10.1016/j.cattod.2020.01.037
Abstract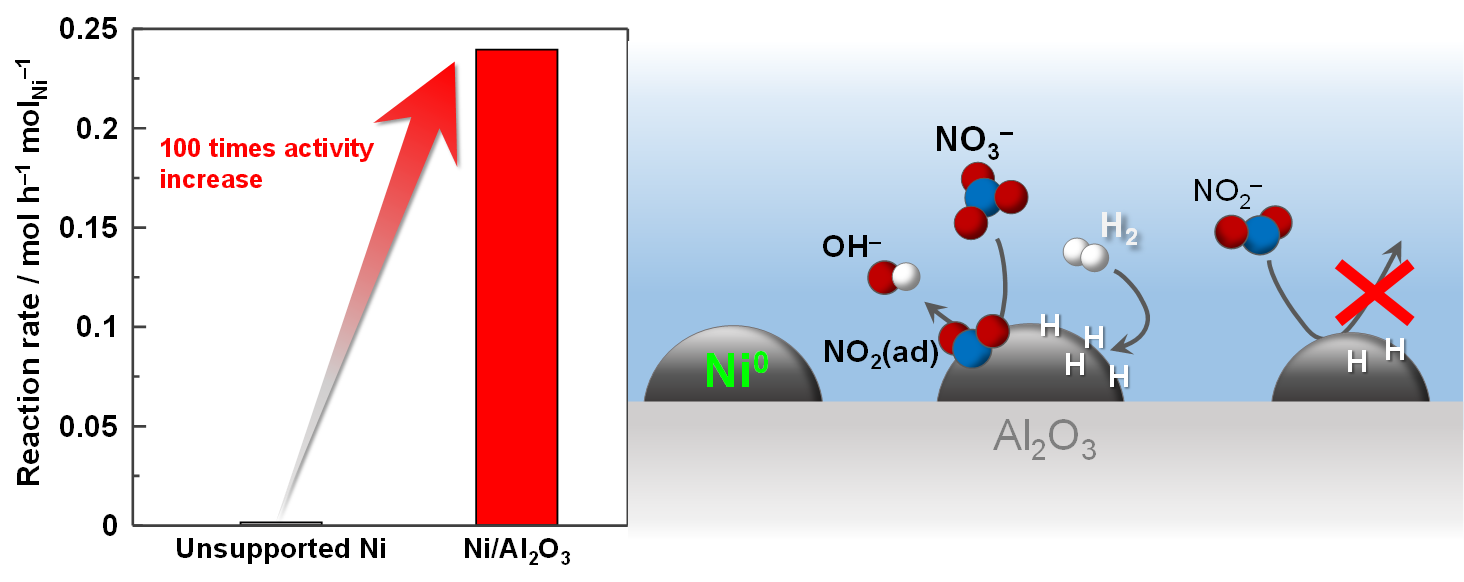
Pollution of groundwater with NO3–is a serious problem in the world. While catalytic reduction of NO3–over Pd-bimetallic catalysts including Cu-Pd and Sn-Pd is a promising method for purification of the groundwater, the use of precious metal is a major obstacle for practical applications. In the present study, we applied Ni/Al2O3for the catalytic reduction of NO3–and compared the catalytic performance with that of unsupported Ni catalyst. The reaction rate over 5 wt.% Ni/Al2O3was about 5 times higher than that of the unsupported Ni catalyst, based on unit weight of catalyst. While the unsupported Ni catalyst was completely deactivated in low partial pressure of H2(= 0.75 atm) and high concentration of NO3–(= 800 ppm), Ni/Al2O3was still active even under less reductive conditions ([NO3–]0 = 800 ppm and P(H2) = 0.5 atm). The unsupported Ni catalyst had the Ni0 particles formed by the reduction of NiO with H2at 310 – 420oC. On the other hand, 5 wt.% Ni/Al2O3possessed the Ni0 particles formed from NiAl2O4on Al2O3by the reduction with H2above 450oC. It is plausible that those Ni0 particles had different properties, giving different catalytic properties. The Ni loadings for Ni/Al2O3had a significant impact on the catalytic properties. The reaction orders with respect to both NO3–and H2were 0.8 for 5 wt.% Ni/Al2O3, while those were 0 and -0.2, respectively, for 10 wt.% Ni/Al2O3. On 10 wt.% Ni/Al2O3, there were two kinds of the Ni0 particles, which were formed by low (310 – 420oC) and high (450oC ~) temperature H2reductions. Unlike the Pd-bimetallic catalysts, the reduction of NO3–over Ni/Al2O3did not proceed through NO2–.
- “Magneli-Phase Titanium Suboxide Nanocrystals as Highly Active Catalysts for Selective Acetalization of Furfural”
Masanori Nagao, Sayaka Misu, Jun Hirayama, Ryoichi Otomo, Yuichi Kamiya
ACS Appl. Mater. Interfaces, ACS Appl. Mater. Interfaces 2020, 12, 2, 2539-2547
DOI:10.1021/acsami.9b19520
Abstract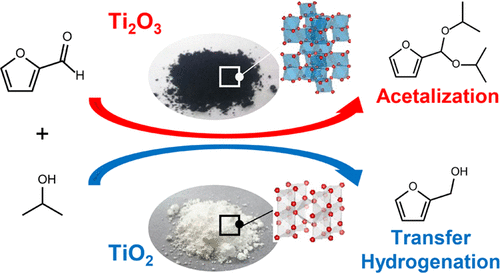
Alongside TiO2, Magneli-phase titanium suboxide having the composition of TinO2n-1is a kind of attractive functional materials composed of titanium. However, there still remain problems to be overcome in the synthesis of titanium suboxide; the existing synthesis methods require high temperature typically over 1000 °C and/or postsynthesis purification. This study presents a novel approach to synthesis of titanium suboxide nanoparticles through solid-phase reaction of TiO2with TiH2. Crystal phases of titanium suboxide were easily controlled by changing TiO2/TiH2molar ratios in a TiO2-TiH2mixed precursor, and a series of titanium suboxide nanoparticles including Ti2O3, Ti3O5, Ti4O7, and Ti8O15were successfully obtained. The reaction of TiO2with TiH2proceeded at a relatively low temperature due to the high reactivity of TiH2, giving titanium suboxide nanoparticles without any postsynthesis purification. Ti2O3nanoparticles and TiO2were applied as solid acid catalysts for reaction of furfural with 2-propanol. Ti2O3showed a high catalytic activity and high selectivity for acetalization of furfural, while TiO2showed only poor activity for transfer hydrogenation of furfural. The difference in catalytic properties is discussed in terms of the acid properties of Ti2O3and TiO2.
- “A reliable method to create adjacent acid-base pair sites on silica through hydrolysis of pre-anchored amide” Editor’s Choice
Wontae Kim, Loida O. Casalme, Taiki Umezawa, Fuyuhiko Matsuda, Ryoichi Otomo, Yuichi Kamiya
Chem. Lett., 49, 2020, 49, 71-74DOI:10.1246/cl.190773
Abstract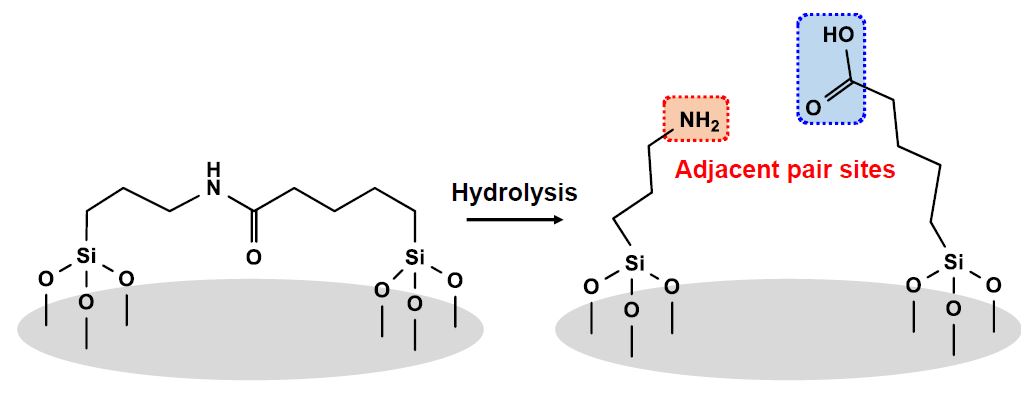
A method to create adjacent acid-base pair sites, which are carboxyl and amino groups, respectively, on silica through hydrolysis of pre-anchored amide is proposed. This method can produce an adjacent acid-base pair sites. The catalyst showed excellent catalytic performance for aldol condensation of 4-nitrobenzaldehyde with acetone, overwhelming the catalyst having only amino group and an acid-base catalyst prepared in a conventional manner.
学会発表
2020年度
- 令和2年度 高難度選択酸化反応研究会シンポジウム (令和3年1月22日(金), オンライン)
(依頼講演) 神谷 ”メタクロレイン選択酸化触媒の作用機構と新触媒開発”
- 触媒学会北海道支部講演会 (12月4日(金), オンライン)
(依頼講演) 神谷 ”硝酸塩で汚染された地下水を浄化するための触媒化学”
- 第36回ゼオライト研究発表会 (11月19日(木)~20日(金), オンライン)
(口頭発表) 中村 ”濃厚ゲルを用いたHf-Betaの短時間合成およびそのルイス酸触媒特性”
- International Symposium on Porous Materials 2020 (11月6日 (金)~7日 (土), オンライン)
(口頭発表) 中村 ”Short-term synthesis of Hf-Beta zeolite from dense precursor gel”
(口頭発表) 黄淵 “Application of Water-resistant Metal-Organic Framework NH2-MIL-53(Al) as a Catalyst Support for Nitrite Reduction in Water”
- 第6回 北大・部局横断シンポジウム(10月19日(月),オンライン)
(ポスター発表) 張“水中1,4-ジオキサンを酸化分解する担持金属触媒の探索”
(ポスター発表) 孔“非化学量論組成を有したリン酸ホウ素触媒を用いた1,2-プロパンジオールの脱水反応”
- 第126回触媒討論会(9月16日(水)~18日(金),オンライン)
(口頭発表) 神谷“アミノ基を導入したMOF(MIL-53)を担体とした担持Pd触媒の亜硝酸イオン還元反応特性”
(口頭発表) 長尾“チタン原子価が酸化チタンの酸性質に及ぼす影響”
- 化学系学協会北海道支部2020年冬季研究発表会(1月28日(火)~29日(水),北海道)
(口頭発表) 加藤“RhおよびAu微粒子内包型イオン交換樹脂を用いた水中硝酸イオンの除去と水素化分解”
(口頭発表) 小山田“SiO2担持MoVOx触媒によるプロパナールの選択酸化”
(口頭発表) 近藤“リン酸ホウ素触媒を用いたグリセリンの気相脱水反応”
(口頭発表) 伊藤“Si/SiC発泡体への酸化セリウムの担持と水中触媒反応への応用”
表彰












