研究業績
研究業績一覧へ
発表論文
2019年
- “Ammonia-treated metal oxides as base catalysts for selective isomerization of glucose in water”
Ryoichi Otomo, Momo Fujimoto, Masanori Nagao, Yuichi Kamiya
Molecular Catalysis, 475 (2019) 110479.
DOI:10.1016/j.mcat.2019.110479
Abstract
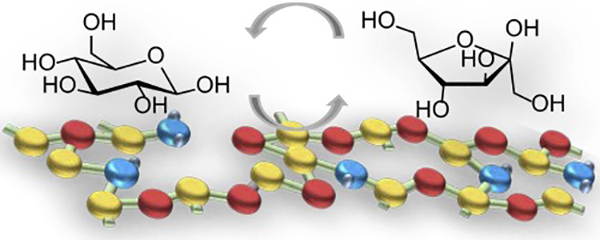
Various metal oxides (MgO, Al2O3, SiO2, TiO2, ZrO2, Nb2O5, and CeO2) were treated with NH3for incorporating nitrogen into the structure of metal oxides. The pristine and NH3–treated metal oxides were used as solid base catalysts for isomerization of glucose to f7ructose in water. SiO2and Nb2O5showed a large increment of nitrogen content by the treatment with NH3. FT-IR study on NH3–treated SiO2revealed that NH3reacted with terminal silanol groups and siloxane bonds to form Si-NH2and Si-NH-Si groups. Meanwhile, nitrogen was incorporated into bulk crystal of Nb2O5, causing amorphization of the crystal. Of all the samples, pristine and NH3–treated MgO showed highest catalytic activity for the isomerization of glucose. However, the selectivity to fructose was low due to subsequent reactions of formed fructose. Catalytic activity of Al2O3and SiO2was increased by the treatment with NH3, while that of the other metal oxides was not affected. Particularly, the catalytic activity of SiO2emerged after the treatment with NH3and was enhanced by increasing the temperature for the treatment up to 800°C. While fructose was consumed by subsequent reactions over MgO, decreasing the selectivity, such reactions of fructose were not noticeable over NH3–treated SiO2. Consequently, NH3–treated SiO2showed higher selectivity to fructose and gave higher carbon balance than MgO.
- “Drastic change in selectivity caused by addition of oxygen to the hydrogen stream for the hydrogenation of nitrite in water over a supported platinum catalyst”
Jun Hirayama, Kei-ichiro Yasuda, Sayaka Misu, Ryoichi Otomo, Yuichi Kamiya
Catalysis Science & Technology, 9 (2019) 4017-4022.
DOI:10.1039/C9CY00999J
Abstract
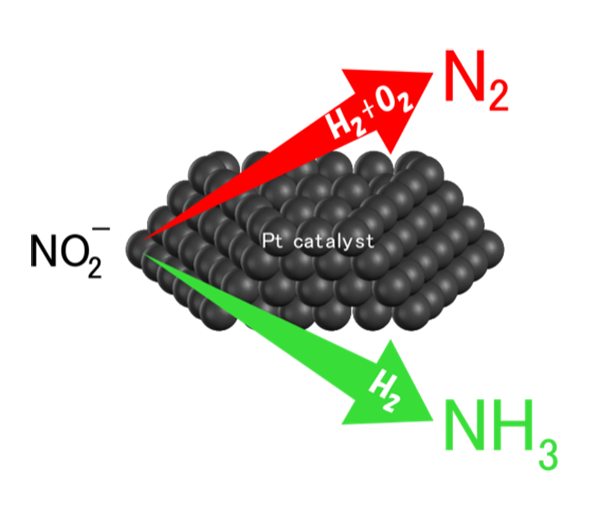
In the present study, we investigated the influence of the addition of O2to the H2reactant stream during the hydrogenation of NO2–in water on the catalytic performances of Al2O3–supported precious metal catalysts including Pd, Pt, Ir, Rh, and Ru with 0.3 mmol g–1of the metal. Pd/Al2O3showed high selectivity for N2irrespective of the presence and absence of O2, and Rh and Ru/Al2O3were inactive towards the hydrogenation of NO2–even in the absence of O2. In contrast, while Pt/Al2O3showed high selectivity for NH3(90%) in the absence of O2(PH2= 0.2 atm and PO2= 0 atm), the product drastically changed to N2with 93% selectivity when O2was added (PH2= 0.2 atm and PO2= 0.1 atm). Since Pt/Al2O3 was completely inactive towards the oxidation of NH3 with O2 in water under the reaction conditions, oxidative decomposition of the formed NH3was not the reason for the high selectivity for N2in the presence of O2. Kinetic analysis of the reaction in the absence and presence of O2and studies on the effects of the Pt size suggested that hydrogen atoms activated on the Pt particles were mainly consumed by O2upon H2O formation in the presence of O2. We concluded that the inactivation of the Pt sites active for NH3formation and furthermore the change in the function of the sites to N2formation caused by the O2addition lead to the drastic change in the selectivity from NH3to N2in the presence of O2.
- “Strong Bronsted acid-modified chromium oxide as an efficient catalyst for the selective oxidation of methacrolein to methacrylic acid”
Shuhei Yasuda, Atsuki Iwakura, Jun Hirata, Mitsuru Kanno, Wataru Ninomiya, Ryoichi Otomo, Yuichi Kamiya
Catalysis Communications, 125 (2019) 43-47.DOI:10.1016/j.catcom.2019.03.020
Abstract
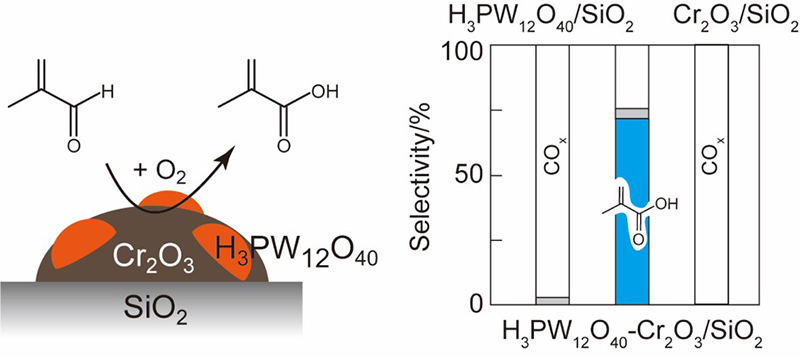
Gas-phase oxidation of methacrolein to methacrylic acid was carried out over an acid-modified Cr2O3/SiO2catalyst. While only total oxidation occurred over bare Cr2O3/SiO2, the acid-modified Cr2O3/SiO2showed catalytic activity for the formation of methacrylic acid. In particular, H3PW12O40strong Bronsted acid was the most effective modifier for improving both activity and selectivity. The interface between Cr2O3and H3PW12O40particles on SiO2appears to be responsible for the formation of active sites for the selective formation of methacrylic acid. The strong Bronsted acid would help the activation of methacrolein through rendering it more electrophilic, which is a key step for the formation of methacrylic acid over the present catalyst.
- “Selective Dehydration of 1,2-Propanediol to Propanal over Boron Phosphate Catalyst in the Presence of Steam”
Ryoichi Otomo, Chiaki Yamaguchi, Daiki Iwaisako, Shun Oyamada, Yuichi Kamiya
ACS Sustainable Chem. Eng., 7 (2019) 3, 3027-3033.DOI:10.1021/acssuschemeng.8b04594
Abstract
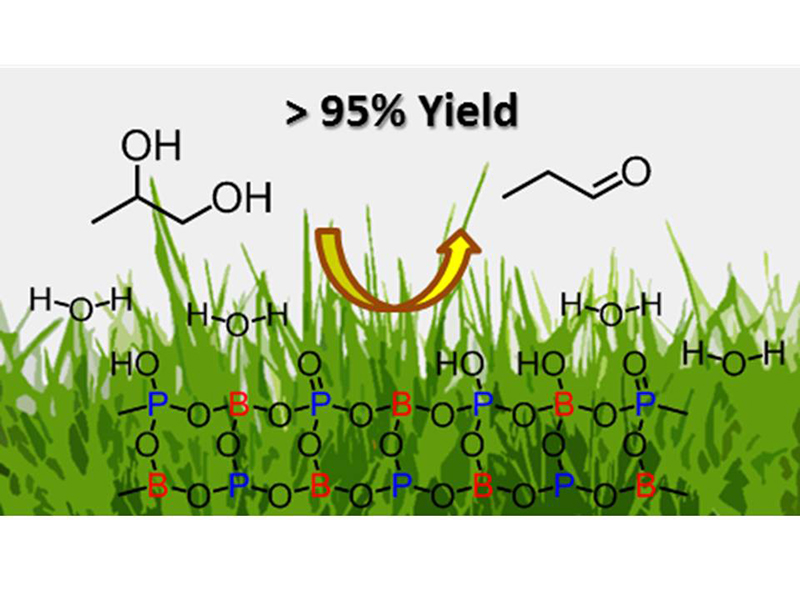
Catalytic properties of metal phosphates were investigated for gas-phase dehydration of 1,2-propanediol to propanal in the presence and absence of steam in the temperature range of 150 – 340 °C. Boron, aluminum, and nickel phosphates showed promising catalytic activity for the dehydration reaction. Especially, boron phosphate showed outstanding catalytic activity at a low reaction temperature and high selectivity to propanal without formation of competitive dehydration products such as acetone and allyl alcohol. The catalytic activity of boron phosphate was remarkably enhanced in the presence of steam co-fed with 1,2-propanediol. The additional steam was also favorable for promoting hydrolysis of dioxolane, which is a by-product formed through acetalization of propanal, resulting in the high yield of propanal over 95%. Boron phosphate showed more durable catalytic activity and much higher yield of propanal than conventional solid acid catalysts such as ZSM-5, silica-alumina and niobium oxide that have been reported to be active for the dehydration of 1,2-propanediol.
- “Octyl and propylsulfonic acid co-fixed Fe3O4@SiO2as a magnetically separable, highly active and reusable solid acid catalyst in water”
Nuryono Nuryono, Ani Qomariyah, Wontae Kim, Ryoichi Otomo, Bambang Rusdiarso, Yuichi Kamiya
Mol. Catal., 475 (2019) 110248 4985-4993.DOI:10.1016/j.mcat.2018.11.019
Abstract
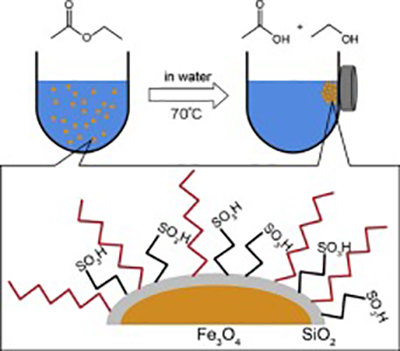
Modifications of propylsulfonic acid-fixed Fe3O4@SiO2with three organosilanes (C2, C8, and phenyl) were investigated to develop a magnetically separable solid acid catalyst active for hydrolysis of ethyl acetate in excess water. Among the organosilanes, triethoxy(octyl)silane was the best modifier for improvement in catalytic activity, with activity of approximately twice that of the unmodified catalyst. The catalytic activity was comparable to those of Cs2.5H0.5PW12O40and H3PW12O40anchored on hydrophobic SBA-15 if compared per acid sites, though the acid strength of sulfonic acid was much weaker than that of H3PW12O40. High hydrophobicity over the surface and around the acid sites created by the octyl group was responsible for the high catalytic activity and the high stability in water achieved. In addition to the improvement in catalytic activity, modification with the octyl group provided high stability for repeated uses of the catalyst in the reaction and there was little decrease in activity over at least four reuses. The catalyst was easily separated from the reaction solution by application of an external magnetic field.
- “The role of steam in selective oxidation of methacrolein over H3PMo12O40“
Shuhei Yasuda, Jun Hirata, Mitsuru Kanno, Wataru Ninomiya, Ryoichi Otomo, Yuichi Kamiya
Appl. Catal. A, 570 (2019) 164-172.DOI:10.1016/j.apcata.2018.11.007
Abstract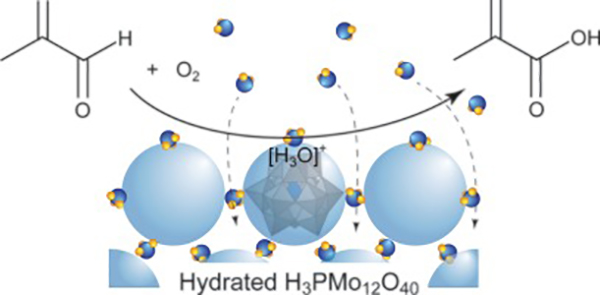
Role of steam in selective oxidation of methacrolein with molecular oxygen over H3PMo12O40catalyst was investigated. Addition of steam to feed gas significantly enhanced both catalytic activity and selectivity to methacrylic acid, which were fivefold and twice increases, respectively, under the optimal steam pressure (PH2O= 0.13 atm). Kinetic analysis demonstrated that the addition of steam caused 200-fold increase in the pre-exponential factor for the formation of methacrylic acid, leading to the significant increase in the activity. The steam in the feed gas varied hydrous state of H3PMo12O40under the reaction conditions, while did not alter redox property, molecular and crystalline structures, and surface area of the catalyst. In the presence of steam at 573 K, three H2O per one H3PMo12O40were absorbed and hydrated protons like [H3O]+were formed in the bulk of H3PMo12O40. Methacrolein was adsorbed on the surface of the hydrous catalyst, but not on anhydrous one at all. Based on the results, it was concluded that activation of methacrolein readily occurred on the catalyst in the presence of steam, leading to the significant increase in the pre-exponential factor. Quantum chemical calculation supported the smooth activation of methacrolein by the reaction with [H3O]+without any transition state.
- “Cs-Beta with an Al-rich composition as a highly active base catalyst for Knoevenagel condensation”
Ryoichi Otomo, Ryota Osuga, Junko N. Kondo, Yuichi Kamiya, Toshiyuki Yokoi
Applied Catalysis A: General, 575 (2019) 20-24.DOI:10.1016/j.apcata.2019.02.014
- “STRAD project for systematic treatments of radioactive liquid wastes generated in nuclear facilities”
Sou Watanabe, Hiromichi Ogi, Yoichi Arai, Haruka Aihara, Yoko Takahatake, Atsuhiro Shibata, Kazunori Nomura, Yuichi Kamiya, Noriko Asanuma, Haruaki Matuura, Toshio Kubota, Noriaki Seko, Tsuyoshi Arai, Tetsuji Moriguchi
Progress in Nuclear, 117 (2019) 103090.DOI:10.1016/j.pnucene.2019.103090
- “Evaluation of Ti Distribution in Zeolite Framework Based on the Catalytic Activity for Alkene Epoxidation”
Xinyi Ji, Yunan Wang, Tsubasa Fujii, Ryoichi Otomo, Junko N. Kondo, and Toshiyuki Yokoi
Chemistry Letters, 48(2019) 1130-1133,.DOI:10.1246/cl.190387
学会発表
2019年度
- 第35回ゼオライト研究発表会 (12月5~6日、東京)
(口頭発表) 中村“フッ素を使ったドライゲルコンバージョン法によるHf-Betaの合成とその触媒特性”
- Internantional Symposium on Porous Material 2019 (11月17日~11月19日、Japan・Tokyo)
(ポスター発表)大友 “Promotional Effect of Fluorine on Incorporation of Hf into Zeolite Beta”
(ポスター発表)黄 “Application of Water-resistant MOF for Catalytic Reaction in Water”
- The 9th East Asia Joint Symposium on Environmental Catalysis and Eco-materials(11月5日~11月8日、China・Yancheng)
(ポスター発表)Philip “Catalytic ozonation of ammonia nitrogen in water over supported noble metal catalysts”
- 第124回触媒討論会 (9月18日~9月20日、長崎)
(口頭発表)劉 “担持ルテニウム 触媒による水中過塩素酸イオン の水素化分解”
(口頭発表)加藤 “金属微粒子を内 包したイオン交換樹脂による水 中からの硝酸イオン除去と水素 化分解”
(口頭発表)中村 “フルフラール類の 移動水素化に高活性を示すHfBetaのポスト合成”
(口頭発表)長尾 “チタン原子価が酸 化チタンの酸触媒特性に及ぼす 影響”
(口頭発表)橋本 “Sn による Fe の部 分置換が SrFeO3のベンゼン酸化 分解活性に与える効果”
- 14th European Congress on Catalysis (8月19日~8月23日、Germany・ Aachen)
(口頭発表)大友 “Adjacent acid-base pair sites on silica surface created by hydrolysis of pre-anchored amide as an excellent catalyst for aldol condensation”
(口頭発表)長尾 “Highly efficient synthesis of titanium suboxide nanoparticles for application as a novel heterogeneous catalyst”
- 第八回Asia Pacific congress on catalysis (APCAT-8)(8月4日~8月7日、タイ・バンコク)
(口頭発表)神谷 “Ceria-supported ruthenium catalyst for rapid reduction of perchlorate for water purification”
(口頭発表)キム “Adjacent acid-base pair sites on silica created by hydrolysis of preanchored amide as an excellent catalyst for aldol condensation”
(口頭発表)Philip “What is the difference in reaction mechanism between in the presence of Co3O4 and MgO for ozonation of ammonia nitrogen in water?”
- 日本化学会北海道支部2019年夏季研究発表会(7月20日、苫小牧)
(口頭発表)中村 “フッ素を添加してポスト合成したHf-Betaのルイス酸触媒特性”
(口頭発表)長尾 “固相合成された低原子価チタン酸化物の触媒特性”
- Southeast Asia Catalysis Conference 2019 (5月23日~5月24日、 Zolg)
(口頭発表)Philip “Pd/CeO2 as a high-performance catalyst for ozonation of ammonio nitrogen in water at neutral”
- 第17回日韓触媒シンポジウム(5月21日、 韓国・済州島)
(口頭発表)神谷 “Ceria-supported Ruthenium Catalyst as a Highly Active Catalyst for Reduction of Perchlorate in Water”
(ポスター発表)大友 “Boron Phosphate as a Highly Active and Selective Catalyst for Dehydration of 1,2-Propanediol”
(ポスター発表)中村 “Fluoride-mediated synthesis of highly active Hf-Beta for MPV reduction”
(ポスター発表)橋本 “Redox properties and catalytic performance of a perovskite-type metal oxide SrFe1-xSnxO3”
(ポスター発表)小船 “Catalytic Reduction of Nitrate in Water over Alumina-Supported Nickel Catalyst”
(ポスター発表)キム “Adjacent acid and base pair on silica created by hydrolysis of preanchored amide as a highly active catalyst for aldol condensation”
(口頭発表)長尾 “Highly efficient synthesis of titanium suboxide nanoparticles for application to novel catalyst material”
- 第123回触媒討論会(3月20日~21日、大阪)
(口頭発表)大友 “ゼオライト上への高活性Hfサイトの形成を促進する添加フッ素の効果”
表彰










