Highly cited papers
(Web of Science, Incites Claviates, 2020.Sep.30)
- ・Ishii et al, Angew. Chem. Int. Ed., 2004, 43, 2702.
- ・Shichibu et al, Small, 2010, 6, 1216.
- ・Kamei et al, Angew. Chem. Int. Ed., 2011, 50,7442.
- ・Shichibu et al, Chem. Commun., 2012, 48, 7559.
- ・Shichibu et al, Nanoscale, 2012, 4, 4125.
- ・Kobayashi et al, J. Am. Chem. Soc., 2013, 135, 16078.
- ・Shichibu et al J. Am. Chem. Soc., 2014, 136, 12892.
- ・Sugiuchi et al, Chem. Commun. 2015, 51, 13519
- ・Bakar et al, Nature Commun., 2017, 8, 576.
- ・Sugiuchi et al, J. Am. Chem. Soc. 2017, 139,1773.
- ・Sugiuchi et al, Angew. Chem. Int. Ed., 2018, 57, 7855
Theory-Directed Ligand-Shell Engineering of Ultrasmall Gold Clusters: Remarkable Effects of Ligand Arrangement on Optical Properties
Rintaro Suzuki, Yuxiang Chen, Yuri Ogawa, Masaki Enokido, Yuichi Kitagawa, Yasuchika Hasegawa, Katsuaki Konishi*, Yukatsu Shichibu*
J. Phys. Chem. Lett., 2025,16,1432
(DOI:10.1021/acs.jpclett.4c03486)
- Ligand-shell engineering of ultrasmall metal clusters is a burgeoning research field aiming to develop cluster-specific properties. However, predicting these properties prior to synthesis is challenging due to their high sensitivity to geometric and/or electronic variations in ultrasmall metal cores, hindering further exploration. In this study, we present a theory-directed ligand-shell design and significant red-shift in absorption of a prolate-shaped [Au8(diphosphine)4Cl2]2+ cluster by synthesizing and characterizing enantiopure octagold clusters bearing chiral BINAP-type ligands [BINAP = 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl]. Crystallographic analysis reveals the predesigned ligand arrangement and twisted gold-core framework. The enantiomeric clusters show significant changes in both absorption and photoluminescence compared with a previous Au8 analogue and exhibit chiroptical signals. Furthermore, theoretical calculations visually unveil the atomic level origins of their optical and chiroptical absorption characteristics. This work not only highlights the effectiveness of ligand-shell engineering in creating unique photophysical properties but also offers a viable, theory-guided strategy for designing and functionalizing ligated metal clusters.
Charge-dependent hierarchical self-assembling of fluorinated gold clusters
Yuki Saito, Duhong Sun, Hayato Kanai, Yasuhiro Ishida, Hideyuki Mitomo, Kuniharu Ijiro, Katsuaki Konishi*
Chem. Commun. 2025,61,1383
(DOI: 10.1039/D4SC02834A)
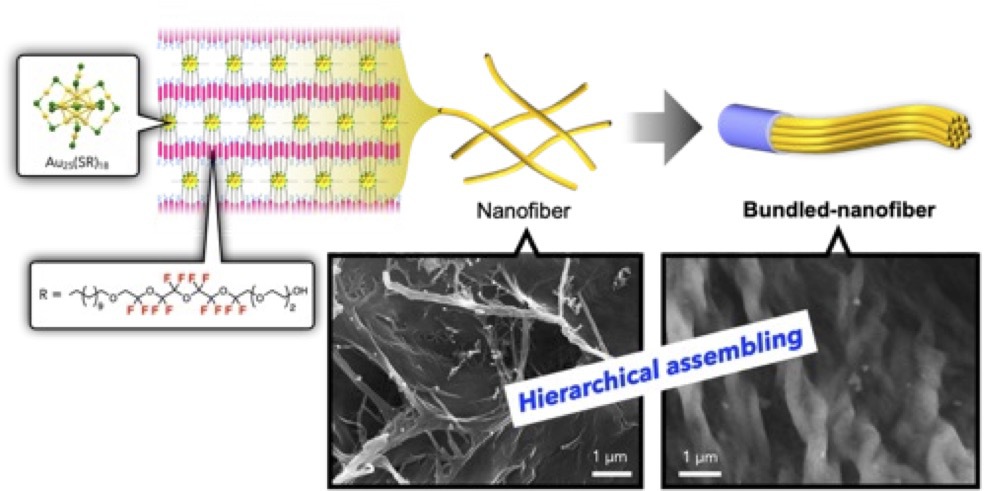 This paper reports the self-assembly behavior of a Au25 cluster with semifluorinated thiolate ligands, highlighting 1) fluorophilic interactions can lead to unexpected anisotropic mesostructures, and that 2) the morphological and hierarchical features of the self-assembly can be tuned through the design of molecular components.The introduction of semifluorinated ligands on Au25 cluster surface results in the formation of hierarchical fibrous nanoassemblies dependent on the inherent charge of the cluster units.
This paper reports the self-assembly behavior of a Au25 cluster with semifluorinated thiolate ligands, highlighting 1) fluorophilic interactions can lead to unexpected anisotropic mesostructures, and that 2) the morphological and hierarchical features of the self-assembly can be tuned through the design of molecular components.The introduction of semifluorinated ligands on Au25 cluster surface results in the formation of hierarchical fibrous nanoassemblies dependent on the inherent charge of the cluster units.
Controlled nanocrystallization of gold nanoclusters within surfactant envelopes: enhancing aggregation-induced emission in solution
Yuki Saito, Ayano Suda, Maki Sakai, Shogo Nakajima,Yukatsu Shichibu, Hayato Kanai, Yasuhiro Ishida*, Katsuaki Konishi*
Chem. Sci. 2024, 15,11885
(DOI: 10.1039/D4SC02834A)
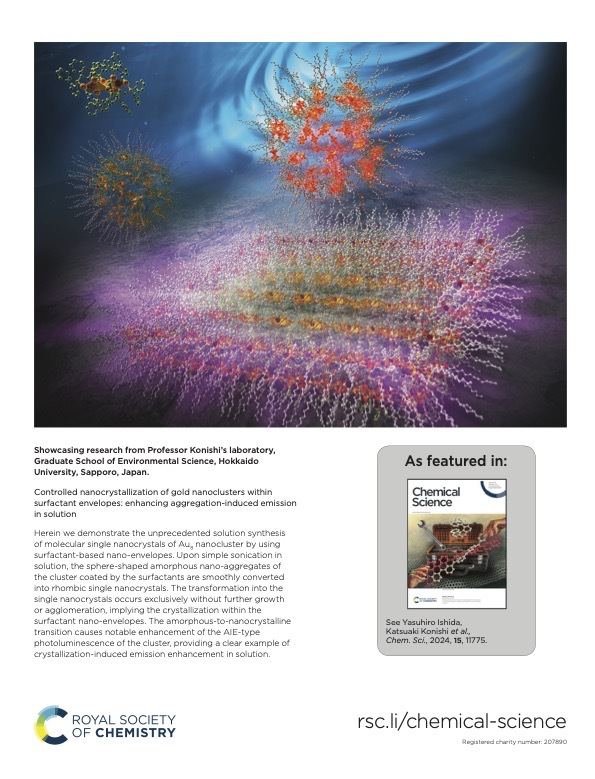 The nanocrystallization of functional molecules has been a subject of recent interest in the current development of nanotechnology. Herein, we report theunprecedented synthesis of singlenanocrystals of a molecular gold nanocluster in a homogeneous solution by using surfactant-based nano-envelopes. The co-assembling of a Au8nanocluster carrying lipophilic phosphine ligands with sodium dodecyl sulfate (SDS) in an aqueous solution results in the formation of sphere-shaped amorphous nano-aggregates coated with the surfactant. Upon sonication, the spherical amorphous aggregates are smoothly shape-shifted into discrete rhombic nanocrystals, which can be tracked by TEM and solution XRD. The transformation into single nanocrystals occurs exclusively without further growth or agglomeration, implying that the crystal growth is restricted within the surfactant nano-envelopes.The robust but flexible nature of thewrapped surfactant is likely responsible for the controlled crystallization. We also demonstrate that the amorphous-to-nanocrystalline transition in solution remarkably enhances the photoluminescence emission from the nanocluster,
The nanocrystallization of functional molecules has been a subject of recent interest in the current development of nanotechnology. Herein, we report theunprecedented synthesis of singlenanocrystals of a molecular gold nanocluster in a homogeneous solution by using surfactant-based nano-envelopes. The co-assembling of a Au8nanocluster carrying lipophilic phosphine ligands with sodium dodecyl sulfate (SDS) in an aqueous solution results in the formation of sphere-shaped amorphous nano-aggregates coated with the surfactant. Upon sonication, the spherical amorphous aggregates are smoothly shape-shifted into discrete rhombic nanocrystals, which can be tracked by TEM and solution XRD. The transformation into single nanocrystals occurs exclusively without further growth or agglomeration, implying that the crystal growth is restricted within the surfactant nano-envelopes.The robust but flexible nature of thewrapped surfactant is likely responsible for the controlled crystallization. We also demonstrate that the amorphous-to-nanocrystalline transition in solution remarkably enhances the photoluminescence emission from the nanocluster,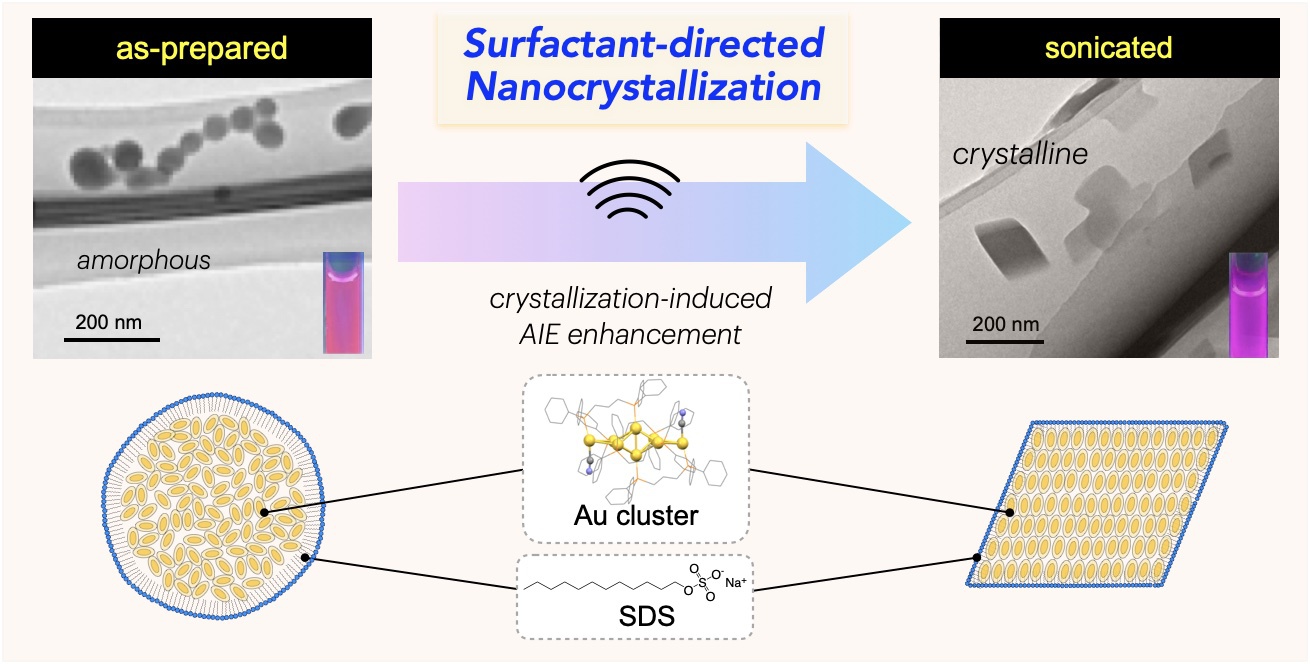 providing a clear example of crystallization-induced emission enhancement.Notably, the obtained nanocrystals showed high stability insolution and retained their shape, size, and PL intensity even after several months, owing to the densely packed surfactant shell. The present surfactant-directed nanocrystallization method may be applicable to other molecular species to contribute to the development of nanocluster science as well as the designed synthesis of nanomaterials.
providing a clear example of crystallization-induced emission enhancement.Notably, the obtained nanocrystals showed high stability insolution and retained their shape, size, and PL intensity even after several months, owing to the densely packed surfactant shell. The present surfactant-directed nanocrystallization method may be applicable to other molecular species to contribute to the development of nanocluster science as well as the designed synthesis of nanomaterials.
Collective Effects of Mutiple Fluorine Atoms Causing π-phillic Characteristic within a Caged Polyoxometalate Framework
Chinatsu Murata, Akari Nakashuku, Yukatsu Shichibu, Katsuaki Konishi*
Chem. Eur. J. 2024, 30, chem.202302328 (DOI:10.1002/chem.202302328)
- Perfluorination brings about distinctive properties arising from the unusual nature of the F element, which have been extensively developed in materials science and chemistry. Herein we report that the construction of F-rich inner space within a hollowed Mo132O372 cage
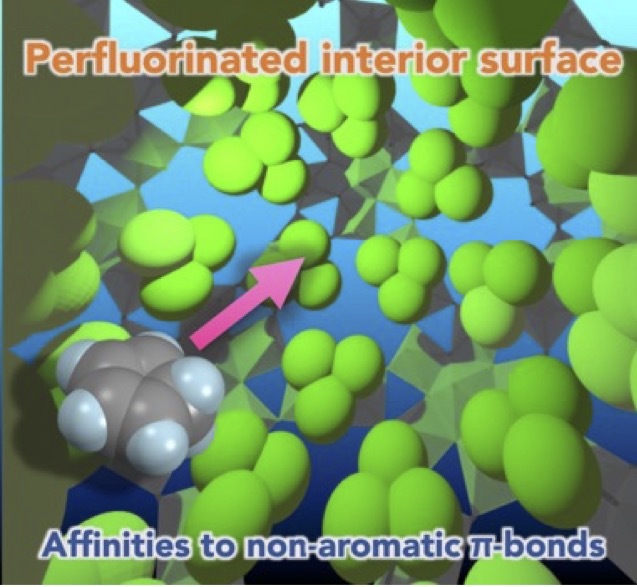 ([Mo132O372(OCOR)30(H2O)72]42-) leads to the emergence of unique guest binding activities inencapsulation. Prominently, the trifluoroacetate-modified cage (R = CF3, 2) having as many as 90 F groups inside favors to trap cyclopentadiene (Cp), which is hardly trapped by the non-fluorinated counterpart (R = CH3, 1). Systematic studies using related hydrocarbons show that the amount of the encapsulated guest is correlated with the unsaturation degree of the guests, implying the involvement of the attractive interaction of the CF3-modified interior wall with the guest π-electron clouds. Control experiments using the semi-fluorinated analogues (R = CF2H, CFH2) reveal that the perfluorination is a critical factor to facilitate the Cp encapsulation by 2, indicating that collective effects of polar C-F bonds spreading over the interior surface, rather than the polarity of the individual C-F bonds, are responsible. We also provide a successful example of the physical molecular confinement within the cage through the “ship-in-a-bottle” Diels-Alder reaction between trapped diene and dienophile.
([Mo132O372(OCOR)30(H2O)72]42-) leads to the emergence of unique guest binding activities inencapsulation. Prominently, the trifluoroacetate-modified cage (R = CF3, 2) having as many as 90 F groups inside favors to trap cyclopentadiene (Cp), which is hardly trapped by the non-fluorinated counterpart (R = CH3, 1). Systematic studies using related hydrocarbons show that the amount of the encapsulated guest is correlated with the unsaturation degree of the guests, implying the involvement of the attractive interaction of the CF3-modified interior wall with the guest π-electron clouds. Control experiments using the semi-fluorinated analogues (R = CF2H, CFH2) reveal that the perfluorination is a critical factor to facilitate the Cp encapsulation by 2, indicating that collective effects of polar C-F bonds spreading over the interior surface, rather than the polarity of the individual C-F bonds, are responsible. We also provide a successful example of the physical molecular confinement within the cage through the “ship-in-a-bottle” Diels-Alder reaction between trapped diene and dienophile.
Anion-πinteraction inside the polyanionic Mo132O372 cage with hydrophobic inner space
Chinatsu Murata, Jaeseob Shin,Katsuaki Konishi*
Chem. Commun., 2023 59, 2441
(DOI: 10.1039/d2cc06875c)
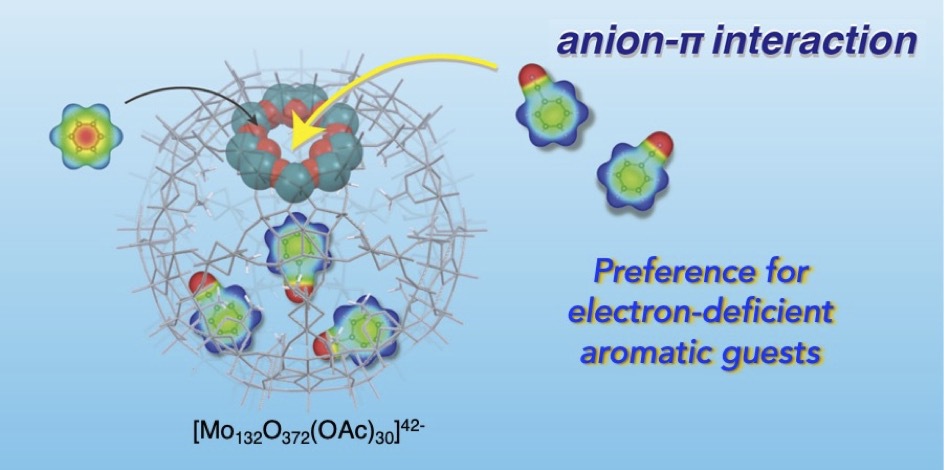
- Anion-π interaction is usually found with highly electron-deficient aromatic rings. Herein we report that such π-interactions can occur, even with mono-substituted benzenes whose electron deficiency is not so high, inside the polyanionic cage of [Mo132O372]42- with acetate-covered interior surface. Systematic studies on the guest preference in the inclusion into the inner space reveal that the polar guests with a strongly electron-withdrawing group (e.g., CN, NO2) show enhanced affinities when compared with less polar guests. Coupled with control experiments using related araliphatic and aliphatic guests, it is implied that the polyanionic nature of the host facilitates to interact with the aromatic ring with a slight δ+ character arising from the π-conjugated electron-withdrawing substituents, boosting the anion-π interactions. This work provides the possibility of the polyanionic caged host to serve as a unique reservoir to accommodate electron-deficient π-compounds.
Ligand-coordinated metal clusters in condensed states: Self-assemblies, crystals, and covalent networks
Yuki Saito, Chinatsu Murata, Mizuho Sugiuchi,Yukatsu Shichibu, Katsuaki Konishi*
Coord. Chem. Rev., 2022 470, 214713
(DOI:10.1016/j.ccr.2022.214713)
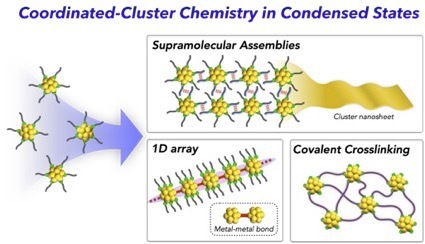
- Coordinated metal clusters composed of a defined metal core and surrounding ligands, as known as atomically precise ligand-protected clusters, have attracted attention because of unique structural fea- tures and properties. Aside from the rapid progress in the research exploring new clusters and elucidating the structure–property relationship at the single-molecule level, interests in the behaviors of the clusters in condensed states have recently emerged. In this review, we focus recent topics on such assembled coordinated metal clusters. First, examples of supramolecular-type assemblies using directional inter- molecular forces are presented, highlighting the emergence of unique nanoscale morphologies. We also show J-aggregate-like or AIE (aggregation-induced emission)-type behaviors associated with the forma- tion of ordered cluster assemblies and crystal polymorph-dependent photoluminescence properties. In addition, recent works concerning 1D cluster arrays bridged by metal–metal bonds and covalently cross- linked multi-cluster systems are shown as the examples of bonded cluster multimers. These examples would provide insights into the rational strategies to construct multi-cluster systems that exhibit unique structures, functions, and properties due to the synergetic effects among the cluster components.
Chiral Gold Clusters with Crosslinking Ligands: Geometric Structures and Chiroptical Activities
Yukatsu Shichibu* and Katsuaki Konishi*
Chem. Nano Mat., 2022, e202200194
(DOI:10.1016/j.ccr.2022.214713)
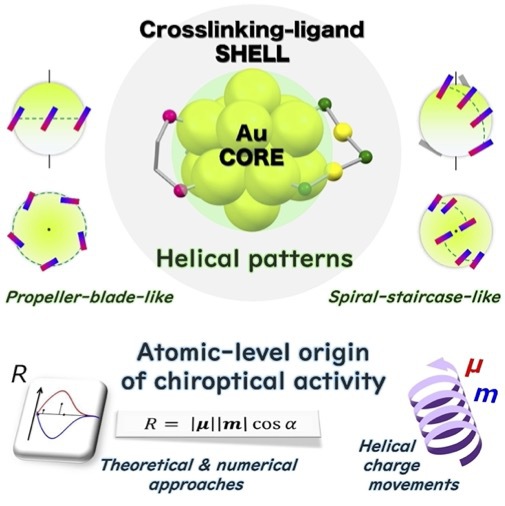

- The geometric diversity of ligand-protected metal clusters has greatly provoked the interest in the chirality of gold clusters bearing crosslinking ligands, which are a subject of active investigations. This review introduces recent geo- metric and computational studies on chiral gold clusters whose metal core is covered by intramolecular crosslinking moieties. Following an overview of the structural construc- tions, the geometric features of the ligand shells and metal cores of chiral gold clusters are elucidated, where anemphasis is placed on the importance of helical patterns of the crosslinking ligands arranged around the gold cores. In addition, insights into the atomic-level origin of chiroptical activity, which cannot be accessed solely through experi- ments, are presented from computational studies using numerical and theoretical approaches, thereby providing a novel methodology based on transition-moment and tran- sition-density analyses.
Self-promoted solid-state covalent networking of Au25(SR)18 through reversible disulfide bonds. A critical effect of the nanocluster in the oxidation processes
Yuki Saito, Yukatsu Shichibu, Katsuaki Konishi*
Nanoscale., 2021,13, 9971–9977
(selected as inside front cover)
- (DOI:10.1039/d1nr01812d)
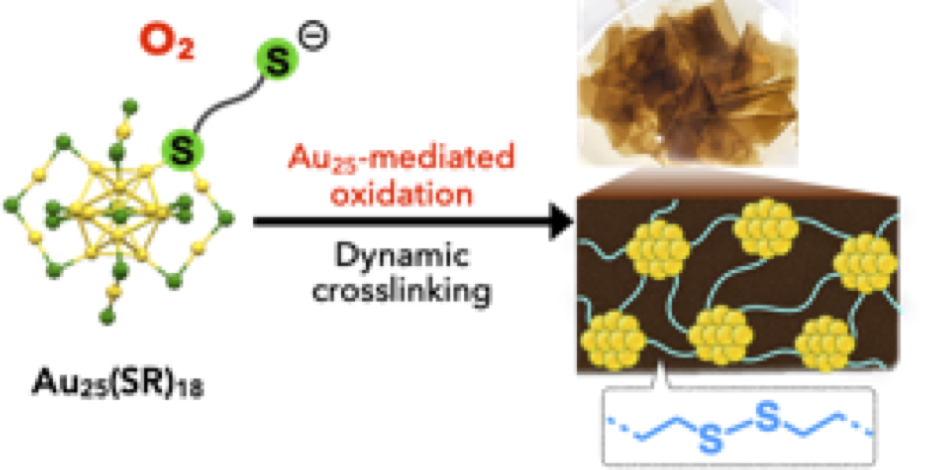

- Covalent crosslinking of ligand-protected gold nanoclusters offers interesting platforms to investigate the properties associated with the synergetic effects of multiple nanoclusters. In this paper, we report the synthesis of covalent networks of [Au25(SR)18]− nanoclusters using reversible disulfide linkages, which was facilitated by the unique capabilities of the nanocluster to mediate oxidation processes. The conventional Au25 synthesis using 1,6-hexanedithiol afforded a soluble oligodisulfide-appended [Au25(SR)18]− monomer possessing uncoordinated anionicthiolate sites at the terminal ends. Upon exposure to O2, the monomer spontaneously underwent intercluster crosslinking in the solid state to give free-standing transparent films, in which the nanoclusters were condensed with the retention of the original Au25 framework.Through studies combined with model experiments, the Au25 cluster was found to be involved inthe O2- mediated radical reactions, promoting the formation of intercluster disulfide linkages. The composition of the films implied the involvement of reversible exchange reactions between disulfide and thiyl radicals, from which it was suggested that solid-state crosslinking occurred in adaptive manners under the control of dynamic covalent chemistry. We also demonstrate that the nanocluster film can serve as a robust and efficient heterogeneous photosensitizer to mediate the generation of singlet oxygen. This work demon- strates a unique aspect of the Au25(SR)18-type nanocluster to mediate oxidation processes as well as the utility of the concept of dynamic covalent chemistry in the bottom-up construction of nanomaterials, which would widen the potential of ligand-protected nanoclusters.
Chiroptical activity of Au13 clusters: experimental and theoretical understanding of the origin of helical charge movements
Yukatsu Shichibu,* Yuri Ogawa, Mizuho Sugiuchi, Katsuaki Konishi*
Nanoscale Adv., 2021, 3, 1005-1011 (selected as back cover)
- Ligand-protected gold clusters with an asymmetric nature have emerged as a novel class of chiral compounds, but the origins of their chiroptical activities associated with helical charge movements in electronic transitions remain unexplored. Herein, we perform experimental and theoretical studies on the structures and chiroptical properties of Au13 clusters protected by mono- and di-phosphine ligands. Based on the experimental reevaluation of diphosphine-ligated Au13 clusters, we show that these surface ligands slightly twist the Au13 cores from a true icosahedron to generate intrinsic chirality in the gold frameworks. Theoretical investigation of a monophosphine-ligated cluster model reproduced the experimentally observed circular dichroism (CD) spectrum, indicating that such a torsional twist of the Au13 core, rather than the surrounding chiral environment by helically arranged diphosphine ligands, contributes to the appearance of the chiroptical response. We also show that the calculated CD signals are dependent on the degree of asymmetry (torsion angle between the two equatorial Au5 pentagons), and provide a visual understanding of the origin of helical charge movements with transition-moment and transition-density analyses. This work provides novel insights into the chiroptical activities of ligand- protected metal clusters with intrinsically chiral cores.
Aggregation-Mode-Dependent Optical Properties of Cationic Gold Clusters: Formation of Ordered Assemblies in Solution and Unique Optical Responses
Mizuho Sugiuchi, Mingzhe Zhang, Yusaku Hakoishi, Yukatsu Shichibu, Noriko Nishizawa Horimoto, Yoshihiro Yamauchi, Yasuhiro Ishida, and Katsuaki Konishi*
J. Phys. Chem. C, 2020,123, 16209-16215
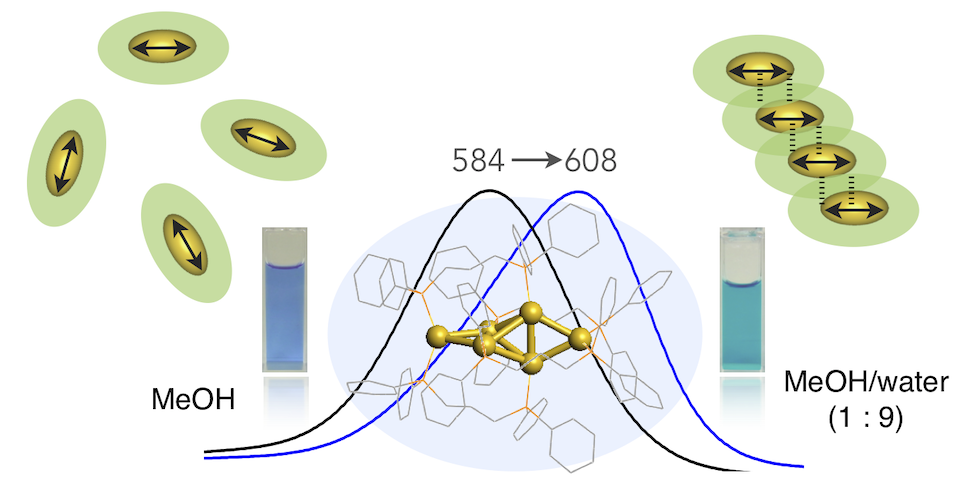 In this Article, we report the results of studies on the aggregation of a diphosphine-ligated [Au6]2+ cluster in aqueous organic solvents, and we highlight the formation of ordered cluster assemblies associated with the J-aggregate-like absorption response and photoluminescence enhancement. Upon aggregation in methanol/water (10/90 v/v), the nitrate salt exhibited a definite red shift of the visible absorption band from the monomeric form. X-ray diffraction profiles of the aggregate in solution gave sharp signals, which revealed the formation of ordered cluster assemblies with particular orientation. The short intercluster distances, as estimated from the diffraction results, imply the interpenetrative packing of the ligand layers, which may contribute to the excitonic coupling of the cluster transition dipoles. The aggregation at the water-rich conditions also caused the enhancement of the near-IR photoluminescence emission, which can be ascribed to the restriction of molecular motion/vibration resulting from the close packing of the cluster molecules in the aggregates. The detailed inspections on the effects of the water contents and counteranions indicated that there are several aggregation modes with different optical features. This work showcases the unique properties of “cluster of clusters” and shows the potential utility of coordinated metal clusters in the design of aggregation-based functional materials.
In this Article, we report the results of studies on the aggregation of a diphosphine-ligated [Au6]2+ cluster in aqueous organic solvents, and we highlight the formation of ordered cluster assemblies associated with the J-aggregate-like absorption response and photoluminescence enhancement. Upon aggregation in methanol/water (10/90 v/v), the nitrate salt exhibited a definite red shift of the visible absorption band from the monomeric form. X-ray diffraction profiles of the aggregate in solution gave sharp signals, which revealed the formation of ordered cluster assemblies with particular orientation. The short intercluster distances, as estimated from the diffraction results, imply the interpenetrative packing of the ligand layers, which may contribute to the excitonic coupling of the cluster transition dipoles. The aggregation at the water-rich conditions also caused the enhancement of the near-IR photoluminescence emission, which can be ascribed to the restriction of molecular motion/vibration resulting from the close packing of the cluster molecules in the aggregates. The detailed inspections on the effects of the water contents and counteranions indicated that there are several aggregation modes with different optical features. This work showcases the unique properties of “cluster of clusters” and shows the potential utility of coordinated metal clusters in the design of aggregation-based functional materials.
Self-assembling-directed Growth and PL Evolution of a Soluble Gold Thiolate Coordination Polymer
Midori Murakami, Riku Matsumine, Takeya Ono, and Katsuaki Konishi*
Chem. Lett, 2020, 49, 1228–1231
(DOI:10.1246/cl.200472)
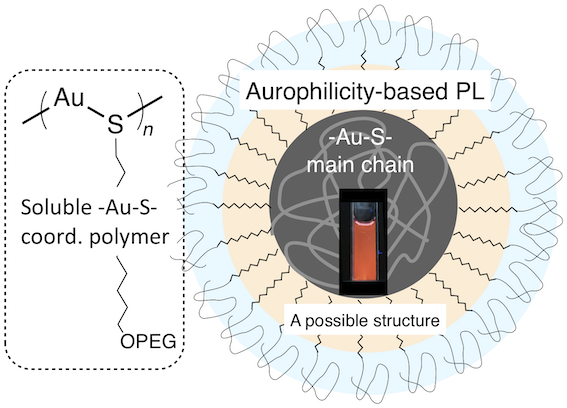
- Herein we report the synthesis of Au(I) thiolate coordination polymers with PEGylated side chains in water, highlighting significant effects of the alkyl spacer to promote the size growth and PL evolution associated with the aurophilic interactions. Such a behavior was specifically observed when a sufficiently long alkyl spacer is implanted, indicating critical roles of the self-organization of the alkyl segments.
Photoluminescence Properties of [Core+exo]‑Type Au6 Clusters: Insights into the Effect of Ligand Environments on the Excitation Dynamics
Yukatsu Shichibu,* Mingzhe Zhang, Takeshi Iwasa, Yuriko Ono, Tetsuya Taketsugu, Shun Omagari, Takayuki Nakanishi, Yasuchika Hasegawa, and Katsuaki Konishi*
J. Phys. Chem. C, 2019, 123, 6934−6939
- The use of multidentate chelating ligands enriches the geometric diversity of coordinated gold clusters, offering much knowledge to benefit the understanding of their unique electronic structures and optical properties. Herein we report different behaviors of [core+exo]-type Au6 clusters bearing C3- and C4-bridged diphosphines (1 and 2), highlighting profound effects of the steric constraints of the ligand environments on the excited-state structural dynamics. Although the Au6 geometries of 1 and 2 in the crystalline states were somewhat different, their absorption spectra in solution were almost similar. However, marked differences were found in the photoluminescence properties; phosphorescent-type emission was dominantly observed for 1, whereas 2 gave both fluorescence- and phosphorescence-type emissions. Theoretical calculations showed that the bridging chains influence the geometries of the Au6 unit in the excited states, leading to the observed differences in emission behaviors.
Unusual Attractive Au-π Interactions in Diacetylene-modified Small Gold Clusters
Mitsuhiro Iwasaki, Yukatsu Shichibu, Katsuaki Konishi*
Angew. Chem. Int. Ed.(communication) 2019, 58, 2443-2447
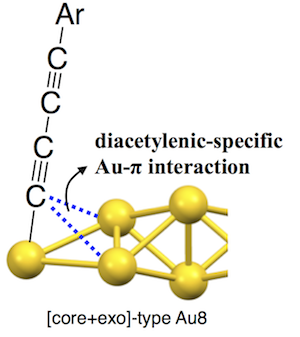
- It is well known that alkynes serve as π-acid to form metal complexes through strong π-coordination bonds. In this work, we highlight unprecedented “attractive” Au-π interactions found in diacetylene-modified [core+exo]-type [Au8]4+ clusters (2). The 4-phenyl-1,3-butadiynyl-modified cluster (2a) showed unusually short Au-Cα distances in the crystal structure, revealing the presence of attractive interactions of the coordinating C≡C moieties with the neighboring bitetrahedral Au6 core, which was further supported by IR and NMR spectra. To note is the fact that such weak interactions were not found in mono-acetylene-modified clusters (1), indicating they are specific for diacetylenic ligands. The Au-π attractive interactions are likely associated with the low π* energy level of the diacetylenic moieties, to which the valence electrons in the gold core may be donated in a back-donation-like manner. 2 showed clear red shifts of > 10 nm with respect to the corresponding mono-acetylenic clusters (1) for the visible absorption bands, indicating the substantial electronic perturbation effects of the Au-π interactions. This work implies potential utility of diacetylenic ligand for the emergence of unique binding motif and cluster structures.
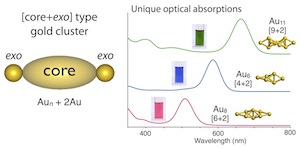
Phosphine-Ligated Gold Clusters with Core+exo Geometries: Unique Properties and Interactions at the Ligand–Cluster Interface
Katsuaki Konishi*, Mitsuhiro Iwasaki, Yukatsu Shichibu
Acc. Chem. Res. 2018, 51, 3125–3133
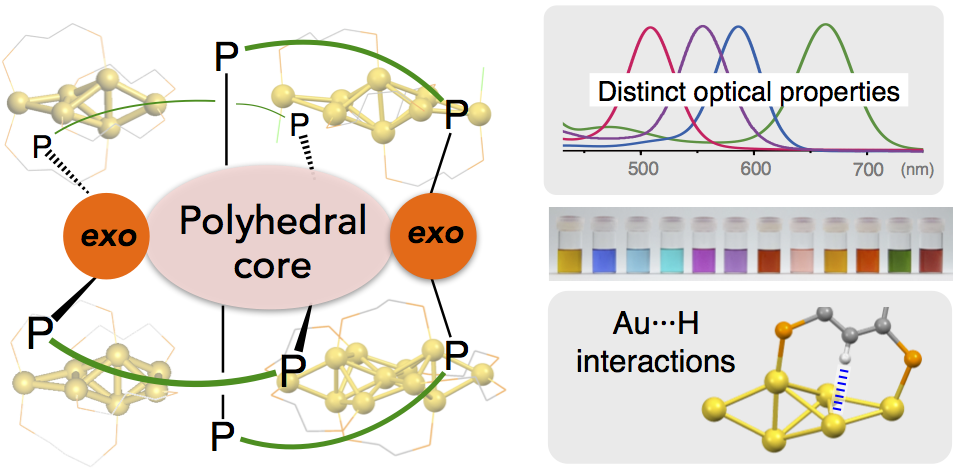
- Over recent years, research on the structures and properties of ligand-protected gold cluster molecules hasgained significant interest. The crystal structure informationaccumulated to date has revealed the structural preference toadopt closed polyhedral geometries, but the use of multidentateligands sometimes leads to the formation of exceptional structures.This Account describes results of our studies on diphosphine-coordinated [core+exo]-type gold clusters featuring extra goldatoms outside the polyhedral cores, highlighting (1) their distinctoptical properties due to the unique electronic structuresgenerated by the exo gold atoms and (2) electronic/attractiveligand−cluster interactions that cause definite perturbation effects on the cluster properties.
- Subnanometer gold clusters with [core+exo]-type geometries (nuclearity = 6, 7, 8, and 11) commonly displayed single absorption bands in the visible region, which are distinct in patterns from those of conventional polyhedral-only homologues. Theoretical studies demonstrated that the exo gold atoms are critically involved in the generation of unique electronic structures characterized by the HOMO−LUMO transitions with dominant oscillator strengths, leading to the appearance of the isolated absorption bands. On the basis of the frontier orbital distributions, the HOMO and LUMO were shown to be localized around the polyhedral cores and exo gold atoms, respectively. Therefore, the HOMO−LUMO transitions responsible for the visible absorptions occur in the core → exo direction. The HOMO−LUMO gap energies showed no clear trends with respect to the nuclearity (size), indicating that the individual geometric features of the inorganic framework primarily govern the clusters’ electronic structures and properties.
- Systematic studies using octagold clusters bearing various anionic coligands revealed that electronic or attractive interactions between the gold framework and ligand functionalities, such as π-electron systems and heteroatoms, cause substantial perturbations of the wavelength of the visible absorption band due to the HOMO−LUMO transitions. Especially, significant red shifts were observed as a result of the electronic coupling with specific π-resonance contributors. It was also found that the orientation of aromatic rings around the inorganic framework is a factor that affects the cluster photoluminescence. These findings demonstrate the utility of the ligand moieties surrounding the gold frameworks for fine-tuning of the optical properties. During these studies, unusual but definite attractive interactions between the gold framework and C−H groups of the diphosphine ligand were found in the hexagold clusters. On the basis of careful crystallographic and NMR analyses, these interactions were deemed as a certain kind of M···H hydrogen bonds, which critically affect the maintenance of the cluster framework. Such unique interaction activities are likely due to the valence electrons in the gold framework, which serve as the hydrogen-bond acceptor for the unfunctionalized C−H groups. Overall, these observations imply the uniqueness of the ligand− cluster interface associated with the partially oxidized gold entities, which may expand the scope of ligand-protected clusters toward various applications.
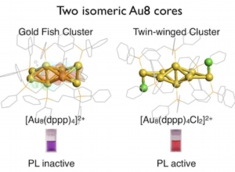
Inherently Chiral Au24 Framework with Double-Helical Hexagold Strands
Mizuho Sugiuchi, Yukatsu Shichibu, Katsuaki Konishi*
Angew. Chem. Int. Ed. (Communication), 2018, 57, 7855–7859
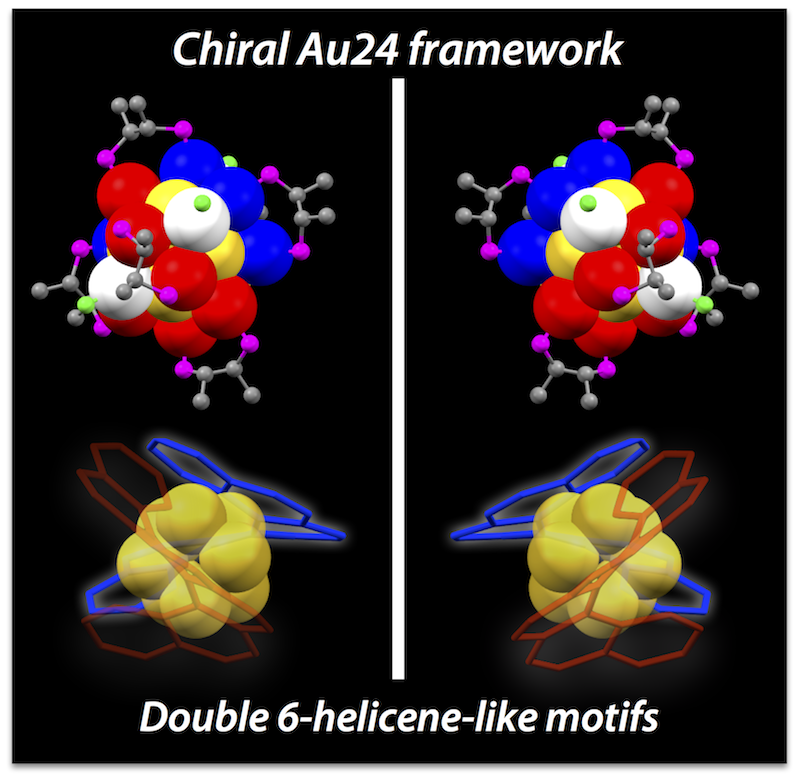
- Chiral gold clusters have attracted attention not only as a unique type of chiral compound but also in relation to the potential applications for chiroptical materials. Herein, we report the use of 2,3-bis(diphenylphosphino)butane enantiomers (chiraphos, L) as chiral auxiliaries results in the preferential formation of an unprecedented Au24 framework with inherent chirality. The crystal structure of [Au24L6Cl4]2+ (1) was revealed to have a square antiprism-like octagold core twinned by two helicene-like hexagold motifs, where the inherent chirality is associated with the helical arrangement. The clusters carrying (R,R)- and (S,S)- diphosphines had right- and left-handed strands, respectively. Circular dichroism spectra showed peaks in the visible to near-IR region, some of which did not coincide with absorption bands, suggesting the enantiomeric Au24 frameworks possess unique chiroptical properties. The Au24 frameworks were highly robust against thermal treatment, which could be attributed to the superatomic concept (18 e– system) and the steric constraint effects of the bridging ligand units. This work will offer novel insight into the unique properties of inherently chiral gold clusters.
Aggregation-induced Fluorescence-to-Phosphorescence Switching of Molecular Gold Clusters
Mizuho Sugiuchi, Junichi Maeba, Nobuyuki Okubo, Munetaka Iwamura, Koichi Nozaki, Katsuaki Konishi*
J. Am. Chem. Soc. 2017 (Communication),139,17731-34.
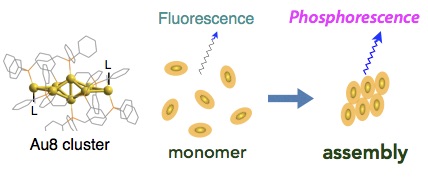
- Aggregation-induced optical responses are ubiquitous among a wide range of organic and inorganic compounds. Here, we demonstrate an unprecedented effect of aggregation on the photoluminescence (PL) profiles of [core + exo]-type [Au8]4+ clusters, which displayed a change in the dominant PL emission mode from fluorescence to phosphorescence-type upon aggregation. In solvents in which cluster molecules are highly soluble and exist as monomers, they displayed single PL bands at ∼600 nm at ambient temperatures. However, in solvents in which cluster molecules are less soluble and cluster aggregation is induced, a new PL band at ∼700 nm also emerged. Lifetime measurements revealed that the PL emissions at ∼600 and ∼700 nm had fluorescence and phosphorescence characters, respectively. Studies of the excitation spectra suggested that organized cluster assemblies were responsible for the lower-energy emission at ∼700 nm and had exceptionally high emission activity. Accordingly, intense phosphorescence-type emissions were observed in the solid state in which the quantum efficiencies were higher by two orders of magnitude than those of the corresponding monomeric forms in solution. This work provides an example of the critical effects of cluster aggregation events on their optical properties and shows the potential of such effects in the design of cluster- based materials with unique functions and properties.
Hydrogen bonds to Au atoms in coordinated gold clusters
Md. Abu Bakar, Mizuho Sugiuchi, Mitsuhiro Iwasaki, Yukatsu Shichibu, Katsuaki Konishi*
Nature Communications, 2017, 8, 576
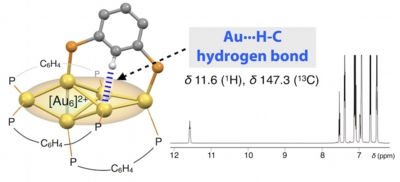
- It is well known that various transition elements can form M···H hydrogen bonds. However, for gold, there has been limited decisive experimental evidence of such attractive interactions. Herein we demonstrate an example of spectroscopically identified hydrogen bonding interaction of C-H units to Au atoms in divalent hexagold cluster ([Au6]2+) decorated by diphosphine ligands. X-ray crystallography reveals substantially short Au-H / Au-C distances to indicate the presence of attractive interactions involving unfunctionalized C-H moieties. Solution 1H and 13C NMR signals of the C-H units appear at considerably downfield regions, indicating the hydrogen-bond character of the interactions. The Au···H interactions are critically involved in the ligand-cluster interactions to affect the stability of the cluster framework. This work demonstrates the uniqueness and potential of partially oxidised Au cluster moieties to participate in non-covalent interaction with various organic functionalities, which would expand the scope of gold clusters.
Ligand-Based Toolboxes for Tuning of the Optical Properties of Subnanometer Gold Clusters
Katsuaki Konishi*, Mitsuhiro Iwasaki, Mizuho Sugiuchi, Yukatsu Shichibu
J. Phys. Chem. Lett., (Perspective) 2016, 7, 4267-4274
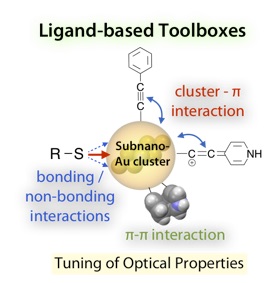
- Recent advances in the crystal structure determination of ligand-protected metal clusters have revealed that their electronic structures and optical features are essentially governed by the nuclearity and geometries of the inorganic frameworks. In this Perspective, we point out the definite effects of the exterior ligand moieties on the properties of small gold clusters. On the basis of systematic experimental studies on the optical properties of Au8 and Au13 clusters with various anionic ligands, it was shown that not only the “through-bond” electronic effects of coordinating atoms but also the nonbonding interaction with neighboring heteroatoms and the electronic coupling with π-systems cause substantial perturbations. We also suggest that the steric rigidity of the ligand environments affects their photoluminescence efficiencies. These findings imply the feasibility of the facile modulation of the cluster properties through the appropriate choice of ligand modules, which may lead to the evolution of novel cluster-based materials with unique properties and functions.
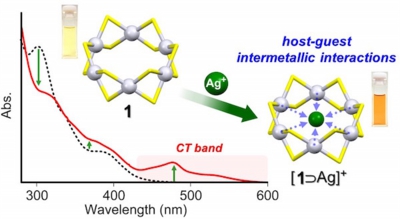
Hexanuclear Platinum(II) Thiolate Macrocyclic Host: Charge-Transfer-Driven Inclusion of a Ag(I) Ion Guest
Yukatsu Shichibu,* Keisuke Yoshida and Katsuaki Konishi*
Inorg. Chem., 2016, 55, 9167-9149 (communication)
- The inclusion of a Ag+ ion by a hexanuclear platinum(II) thiolate macrocycle in solution was demonstrated, and the inclusion structure was determined by X- ray crystallography. Unique host−guest intermetallic interactions driven by charge transfer were elucidated by optical absorption spectroscopy and theoretical calculations.
Facile modulation of optical properties of octagold clusters through the control of ligand-mediated interactions
Mitsuhiro Iwasaki, Naoki Kobayashi, Yukatsu Shichibu and Katsuaki Konishi*
Phys. Chem. Chem. Phys. 2016, 18, 19433-19439
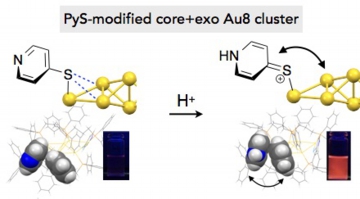
- In the recent development of structurally defined ligand-stabilized gold clusters, it has been revealed that not only the inorganic units but also the surrounding organic ligands substantially affect their electronic/optical properties. In this work, a series of core + exo type Au8 clusters decorated by dppp (Ph2P(CH2)3PPh2) and arylthiolate ligands ([Au8(dppp)4(SR)2]2+, 1–5) were synthesized, and their optical properties were studied in order to gain insights into the perturbation effects of the organic ligands. 1–5 showed visible absorption and photoluminescence emission bands at longer wavelengths compared to their chloro- and acetylide-modified analogues, suggesting the contribution of weak non-bonding interactions of the Au framework with the ligand heteroatoms. Upon acid treatment, 2- and 4-pyridinethiolate clusters (R = Py, 2 and 4) showed larger red shifts of the absorption and emission bands than the 3-pyridyl isomer (3), implying the involvement of the resonance structures of the SPy units. On the other hand, all regioisomers (2–4) showed large photoluminescence enhancements upon pyridine protonation. X-ray crystallographic and NMR analyses of 4 and its protonated form (40) showed that the electron-deficient pyridinium rings of 40 form p-stacks with neighbouring phenyl groups of dppp, suggesting that the orientation of the surface aromatics is a plausible factor governing the emission efficiency. These observations provide examples of successful modulation of optical properties of small gold clusters through the electronic and/or steric perturbation by the proximal organic ligands, highlighting the importance of the ligand design in the fine tuning of cluster properties directed for optical chemosensors and luminescent materials..
Cluster-π electronic interaction in a superatomic Au13 cluster bearing σ-bonded acetylide ligands
Mizuho Sugiuchi, Yukatsu Shichibu, Takayuki Nakanishi, Yasuchika Hasegawa and Katsuaki Konishi*
Chem. Commun., 2015, 51, 13519 - 13522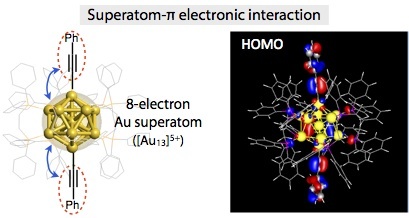
(DOI: 10.1039/C5CC04312C)
- An organometallic Au13 cluster having two σ-bondedacetylide ligands was synthesized and its structure was determined by X-ray crystllography. Absorption spectral studies indicated the presence of the electronic coupling between the superatomic Au13 core and the acetylide π-orbitals, which was supported by theoretical considerations.
Impact of Skeletal Isomerization of Ultrasmall Gold Clusters on Electrochemical Properties: Voltammetric Profiles of Nonspoked Octanuclear Clusters.
Yutaro Kamei, Neil Robertson, Yukatsu Shichibu, Katsuaki Konishi*
J. Phys. Chem. C, 2015, 119, 10995−10999
(DOI: 10.1021/jp511296f)
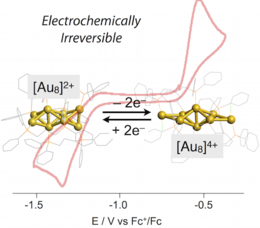 Electronic properties of ultrasmall gold clusters with defined nuclearity and geometrical structures have been a recent subject of interest not only with respect to the concept of molecularity but also because of their potential applicability as nanomaterials and catalysts. In this work, the electrochemical properties of dppp-protected octagold clusters ([Au8L4]n+ (L = dppp, n = 2 (1) and 4 (2), dppp = Ph2P(CH2)3PPh2) with charge-dependent geometrical structures were investigated. Unlike conventional sphere-like centered clusters held by multiple spokes, the non-spoked Au8 clusters displayed irreversible electrochemical profiles for the two-electron redox interconversion between 1 and 2, exhibiting a wide energy gap between the redox couples. This electrochemical irreversibility could be attributed to the significant alteration of electronic structures associated with the redox-coupled isomerization of the non-spoked cluster structures. In addition, we show that the coordinative interaction of Cl− anions with the Au8 clusters notably affects both reduction and oxidation courses, providing an example of coordination-coupled electron transfer events.
Electronic properties of ultrasmall gold clusters with defined nuclearity and geometrical structures have been a recent subject of interest not only with respect to the concept of molecularity but also because of their potential applicability as nanomaterials and catalysts. In this work, the electrochemical properties of dppp-protected octagold clusters ([Au8L4]n+ (L = dppp, n = 2 (1) and 4 (2), dppp = Ph2P(CH2)3PPh2) with charge-dependent geometrical structures were investigated. Unlike conventional sphere-like centered clusters held by multiple spokes, the non-spoked Au8 clusters displayed irreversible electrochemical profiles for the two-electron redox interconversion between 1 and 2, exhibiting a wide energy gap between the redox couples. This electrochemical irreversibility could be attributed to the significant alteration of electronic structures associated with the redox-coupled isomerization of the non-spoked cluster structures. In addition, we show that the coordinative interaction of Cl− anions with the Au8 clusters notably affects both reduction and oxidation courses, providing an example of coordination-coupled electron transfer events.
[Au7]3+: A Missing Link in the Four-Electron Gold Cluster Family
Yukatsu Shichibu, Mingzhe Zhang, Yutaro Kamei, Katsuaki Konishi*
J. Am. Chem. Soc. (communication), 2014, 136, 12892-12895
(DOI: 10.1021/ja508005x)
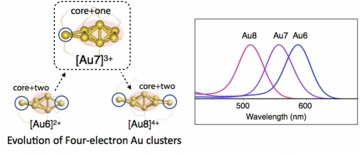 Ligand-stabilized ultrasmall gold clusters offer a library of diverse geometrical and electronic structures. Among them, clusters with four valence electrons form an exceptional but interesting family because of their unique geometrical structures and optical properties. Here, we report a novel diphosphine-ligated four-electron Au7 cluster (2). In good agreement with previous theoretical predictions, 2 has a “core+one” structure to exhibit a prolate shape. The absorp-tion spectrum showed an isolated band, similar to the spectra of Au6 and Au8 clusters with “core+two” structures. TD-DFT studies demonstrated that the attachment of only one gold atom to a polyhedral core is sufficient to generate unique elec-tronic structures and characteristic absorptions. The present result fills the missing link between Au6 and Au8 in the four-electron cluster family, showing that the HOMO–LUMO gap increases with increasing nuclearity in the case of the tetrahe-dron-based “core+exo” clusters.
Ligand-stabilized ultrasmall gold clusters offer a library of diverse geometrical and electronic structures. Among them, clusters with four valence electrons form an exceptional but interesting family because of their unique geometrical structures and optical properties. Here, we report a novel diphosphine-ligated four-electron Au7 cluster (2). In good agreement with previous theoretical predictions, 2 has a “core+one” structure to exhibit a prolate shape. The absorp-tion spectrum showed an isolated band, similar to the spectra of Au6 and Au8 clusters with “core+two” structures. TD-DFT studies demonstrated that the attachment of only one gold atom to a polyhedral core is sufficient to generate unique elec-tronic structures and characteristic absorptions. The present result fills the missing link between Au6 and Au8 in the four-electron cluster family, showing that the HOMO–LUMO gap increases with increasing nuclearity in the case of the tetrahe-dron-based “core+exo” clusters.
Phosphine-coordinated Pure-Gold Clusters: Diverse Geometrical Structures and Unique Optical Properties / Responses.
Katsuaki Konishi
Struct. Bonding (review), 2014, 161, 49-86
DOI: 10.1007/430_2014_143
- Free Download of Author Version → Hokkaido Univ Library (HUSCAP)
- Synthetic techniques, geometrical structures, and electronic absorption spectra of phosphine-coordinated pure-gold molecular clusters (PGCs) accumulated over 40 years are comprehensively collected especially for those with unambiguous X-ray crystal structures available. Inspection of the electronic absorption spectra from geometrical aspects reveals that their optical properties are highly dependent on the cluster geometries rather than the nuclearity. Recent examples of unusual clusters that show unique color / photoluminescence properties and their utilization for stimuli-responsive modules are also presented.
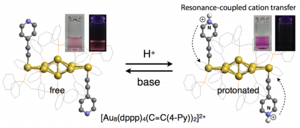
Protonation-induced Chromism of Pyridylethynyl-appended [core+exo]-type Au8 Clusters. Resonance-coupled Electronic Perturbation through π-Conjugated Group.
Featured in JACS Spotlights (link)
Naoki Kobayashi, Yutaro Kamei, Yukatsu Shichibu, Katsuaki Konishi*
J. Am. Chem. Soc. (communication), 2013, 135, 16078-16081
DOI: 10.1021/ja4099092
- A series of [core+exo]-type Au8 clusters bearing two alkynyl ligands on the exo gold atoms ([Au8(dppp)4(C≡CR)2]2+, 2–6) were synthesized by the reaction of [Au8(dppp)4]2+ (1) with alkynyl anions. Although the C≡C moieties directly attached to the Au8 units did not affect the optical properties arising from intracluster transitions, the pyridylethynyl-bearing clusters (4–6) exhibited reversible visible absorption and photoluminescence responses to protonation/deprotonation events of the terminal pyridyl moieties. The chromism behaviors and proton-binding constants of these clusters were highly dependent on the relative position of the pyridine nitrogen atom, such that the 2-pyridyl (4) and 4-pyridyl (6) isomers showed more pronounced responses than the 3-pyridyl isomer (5). These results suggest that the resonance-coupled movement of the positive charge upon protonation is involved in the optical responses, where the formation of extended charged resonance structures causes significant perturbation effects on the electronic properties of the Au8 unit and also contributes to the high binding affinities.
Electronic Properties of [Core+exo]-type Gold Clusters: Factors Affecting the Unique Optical Transitions
Yukatsu Shichibu, Katsuaki Konishi
Inorg. Chem., 2013, 52, 6570–6575
DOI:10.1021/ic4005592
- Optical properties of [core+exo]-type gold clusters, which show an unusual isolated visible absorption, were studied from experimental/theoretical aspects. Through studies on three clusters of different nuclearity, we have shown the absorption properties are dependent on the cluster geometry rather than the nuclearity. Theoretical results indicated that, for all the clusters, the exo gold atoms are critically involved in the generation of the HOMO and LUMO bands responsible for the appearance of the visible absorptions.
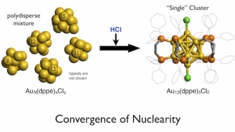
Facile synthesis and optical properties of magic-number Au13 clusters
Yukatsu Shichibu, Kai Suzuki, Katsuaki Konishi
Nanoscale, 2012, 4, 4125-29
DOI:10.1039/C2NR30675A
- Synthesis of molecular gold clusters through the post-synthetic scheme involving HCl-promoted nuclearity convergence was examined with various phosphine ligands. Systematic studies with a series of bis(diphenylphosphino) ligands (Ph2P-(CH2)m-PPh2) using electrospray ionization mass spectrometry (ESI-MS) and electronic absorption spectroscopy demonstrated that the use of dppp (m = 3), dppb (m = 4) and dpppe (m = 5) as the ligand resulted in the formation of [Au13P8Cl4]+ type clusters, whereas [Au13P10Cl2]3+ type cluster was formed with dppe (m = 2). The cluster species did not survive the HCl treatment step when monophosphines PPh3, PMe2Ph, and POct3 were employed, but [Au13(POct3)8Cl4]+ was isolated as a minor product in the NaBH4 reduction of Au(POct3)Cl in aqueous THF. Electronic absorption and photoluminescence studies of a series of Au13 clusters revealed that their optical properties are highly dependent on the phosphine/chloride composition ratio, but are far less so on the phosphine structure.
Unique [core+two] structure and optical property of dodeca-ligated undecagold cluster: Critical contribution of the exo gold atoms to the electronic structure
Yukatsu Shichibu, Yutaro Kamei, Katsuaki Konishi
Chem. Commun., 2012, 48, 7559-61
DOI:10.1039/C2CC30251A
- A novel dodeca-ligated undecagold cluster having a nonagold core plus two exo-attached gold atoms was synthesized. Unlike the conventional icosahedron-based “core-only” isomer, the [9+2] cluster showed an intense visible absorption band. Theoretical calculation showed the involvement of the exo gold atoms in the generation of the unique electronic structure
Generation of Small Gold Clusters with Unique Geometries through Cluster-to-Cluster Transformations: Octanuclear Clusters with Edge- sharing Gold Tetrahedron Motifs
Yutaro Kamei, Yukatsu Shichibu, Katsuaki Konishi
Angew. Chem. Int. Ed., 2011, 50, 7442-45
DOI: 10.1002/anie.201102901
- A post-synthetic approach using a diphosphine ligand allowed the generation of two novel Au8 clusters with unique core geometries containing edge-shared gold tetrahedron motifs. These Au8 clusters with tetrahedron-based isomeric cores showed discrete absorption bands in the visible region to exhibit characteristic colors, and their optical properties were strictly dependent on the core geometries associated with the charge states.
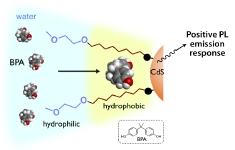
Surface-Functionalized CdS Clusters with Recognition Sites near the Interface: Selective Luminescence Response to Lipophilic Phenols
Katsuaki Konishi, Eri Takase, Naoto Fukunaga
Langmuir, 2011, 27, 1332-35(invited)
DOI: 10.1021/la103956m
- A series of water-soluble cadmium sulfide clusters bearing an alkyl-chain layer between the inorganic core and the outer PEG layer were synthesized by the ligand exchange reaction of Cd10S4(SPh)12 with thiols functionalized by an N-(ω-PEGylated alkyl) amide moiety. The photoluminescence titration experiments in aqueous media revealed that the clusters with a sufficiently hydrophobic inner environment exhibit definite emission enhancements upon the addition of bisphenol A or 4-nonylphenol. The dramatic effect of the alkyl-chain length on the emission responses demonstrated that the hydrophobic layer around the inorganic surface serves as guest binding sites to facilitate the access of the lipophilic phenols near the organic-inorganic interface. A marked preference for the lipophilic phenols over related compounds, such as methylated bisphenol A, long-chain n-alkanol, and non-lipophilic phenols, was observed in the emission responses of the ‘hydrophobic’ cluster, suggesting that not only hydrophobic interaction but also attractive force involving the phenolic OH group contribute to the positive responses. The results of control experiments and IR studies indicated that the hydrogen bonding interaction between the phenolic OH group and the amide group in the surface organic units is responsible for the positive emission responses. The present work shows that the precise tuning of the molecular recognition environments near the organic-inorganic interface is useful to develop guest-specific functions.
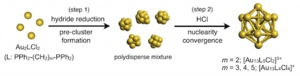
HCl-induced Nuclearity Convergence in Diphosphine-protected Ultrasmall Gold Clusters: A Novel Synthetic Route to "Magic-Number" Au13 Clusters
Yukatsu Shichibu, Katsuaki Konishi
Small, 2010, 6, 1216-1220
DOI: 10.1002/smll.200902398
- A magic-number Au13 cluster with icosahedral geometry was exclusively generated from a polydisperse mixture of ultrasmall 1,2-bis(diphenylphosphino)ethane -coordinated gold clusters upon treatment with HCl, which acts as an efficient promoter.
 Japanese
Japanese

 HOME
HOME English
English